|
|
|
|
Introduction to the gobies
|
| The gobies
are the largest family of reef fishes and
account for a major fraction of the world's
tropical marine fish fauna. There are well
over a hundred Caribbean species, and doubtless
a few more to be described. In addition, there
are numerous cryptic species among the gobies
in the western Atlantic (populations with
sharply divergent DNA sequences that are usually
allopatric, but can be sympatric). Although
almost always small and inconspicuous, gobies
occur in large numbers in all reef-associated
habitats. There are over 30 regional genera
and, unfortunately, many groupings of closely
related species that make species-level larval
identifications particularly challenging.
I have managed to identify and include in
this guide the larvae of almost all of the
shallow-water goby genera of the region; a
few deep-water genera remain unknown. |
|
|
| The
great taxonomic diversity of gobies is certainly
reflected in their early life history
stages. Larval gobies exhibit the full range of
larval sizes at transition, from about 4 mm to almost
30 mm SL. In general, however, they are small and
nondescript with a long, narrow, and thin body.
They tend to have small to medium-sized terminal
mouths, small heads without spines, and slender
flexible spines in the fins. They can be recognized
most readily by their two separated dorsal fins
with the first having only a few spindly spines.
In addition, they often have fused pelvic fins and
typically light markings. The basic marking pattern
for goby larvae is a ventral midline series of melanophores:
at the isthmus, pelvic-fin base, anal-fin base,
and caudal peduncle, along with a variety of other
small melanophores. Some larval gobies also have
markedly narrowed and tilted eyes. Since Caribbean
goby genera are often quite speciose and the larvae
only become distinct during transition or even later,
some groups will certainly require DNA
testing for the identification of individual
larvae to the species level. |
|
How
to divide the gobies up?
|
|
Since there are too many Caribbean species to
deal with on one webpage, the goby family needs
to be subdivided, a task that has tested more
than a few fish taxonomists and can be quite frustrating.
A variety of divisions have been proposed in the
past, none of which have been satisfactory and
certainly none have been backed up by strong phylogenetic
evidence. Gobies are highly variable in morphology
and genetics and deep phylogenies so far are quite
elusive. Suffice to say, some traditional separations
based on the state of fusion of the pelvic fins
and the presence or absence of pores and scales
are not reflective of true relationships. Indeed,
the state of the pelvic fins can be variable among
larvae of obviously close relatives. The evolutionary
loss of pores and scales is an individual adaptation
and not likely a shared attribute among relatives.
Larval markings in gobies are often sparse and
the basic patterns are generally shared by unrelated
groupings and do not fall out in manageable blocks.
Since genetic relatedness is an unwieldy method
to subdivide larval forms in a group this complex,
I have tried to arrange the groups in a form that
makes it easier to navigate. The basic separations
I use are six vs. seven first-dorsal-fin spines,
the short and long-fin groups, i.e. the increasing
number of median-fin rays, and the fusion state
of the pelvic fins.
The number of dorsal-fin spines, six vs. seven,
is not always easy to see on larvae, but is consistent
enough to be useful and seems a natural separation
among gobies. The number of dorsal and anal-fin
soft rays is somewhat consistent within similar-appearing
larvae and the "long-fin" gobies with
more than 11 second-dorsal and anal-fin elements
are usually easy to distinguish from the "short-fin"
gobies, typically with 9, 10, or 11 second-dorsal
and anal-fin elements. Lastly, although pelvic-fin
states can be phylogenetically labile, the state
of fusion is often an obvious visible attribute
of goby larvae and the division of the pelvic
fins seems to be a characteristic of a set of
goby species as well as the allied gobioids, the
eleotrids and ptereleotrids.
|
|
Group 1:
|
Short-fin gobies 1 (six-spined)
|
|
Bathygobius, Lophogobius, Priolepis, Sicydium,
Awaous
|
|
|
Group 2:
|
Short-fin gobies 2 (six-spined)
|
|
Coryphopterus and Lythrypnus
|
|
|
Group 3:
|
Short-fin gobies (seven-spined)
|
|
Barbulifer, Elacatinus, Tigrigobius, Gobiosoma,
Risor, Ginsburgellus
|
|
|
Group 4:
|
Long-fin gobies
|
|
Evermannichthys, Ctenogobius, Gnatholepis,
Nes, Evorthodus, Ctenogobius, Bollmannia, Gobionellus,
Oxyurichthys, Gobioides, Microgobius, Palatogobius
(Ptereleotris included)
|
|
|
Group 5:
|
Divided-pelvic-fin gobies
|
|
Psilotris, Pycnomma, Gobulus, Chriolepis
|
|
|
|
|
|
|
|
|
|
|
|
| GOBIES
OF THE CARIBBEAN |
|
|
there are a total of about 120 Caribbean species
|
|
| Sources
for taxonomy and fin-ray counts include Fishbase,
Gobiidae.com,
the
FAO key, Randall's Caribbean Reef Fishes,
Peterson Field Guides Atlantic Coast Fishes,
Bohlke and Chaplin's Fishes of the Bahamas,
McEachran and Fechhelm's Fishes of the Gulf
of Mexico, Richards' Early Stages
of Atlantic Fishes goby chapter, Bohlke
and Robins' Western Atlantic Gobies
(PANS Phila.) and their Revision of ...
Coryphopterus and specific literature. |
| |
|
|
|
| |
|
Quick
Key to Genera: in order of increasing anal-fin
elements |
|
| |
|
|
|
| |
|
|
|
|
|
|
|
skip to page with
|
|
|
|
|
|
|
|
|
|
|
Larval Gobies
|
|
| Goby larvae
are typically the most abundant larvae collected in most
reef fish larval collections, both in diversity and often
in total numbers. Indeed, Ctenogobius
saepepallens, the dash goby, is the most frequently
occurring larval type in my Panama collections, followed
closely by the bridled gobies, Coryphopterus
spp. |
|
| Since the
process of elimination is critical to the identification
of larval gobies, the diversity within this group makes
for some difficulty in species ID. A variety of other
factors add to the complexity of identifying goby larvae:
|
|
|
|
|
|
Melanophore patterns
|
| |
| The larval
melanophore patterns within the family tend to be conservative,
with many larval types sharing a sparse basic pattern
of a ventral midline series of melanophores: at the isthmus,
pelvic-fin base, anal-fin base, and caudal peduncle. Melanophore
patterns can be quite variable within types- many individuals,
especially earlier-stage larvae, are missing one or a
few of the standard complement for their type. |
| |
| Melanophores
can be contracted, appearing as discrete dots, or
expanded into either complex dendritic star-shapes
or linear forms. Linear melanophores often merge
with adjacent melanophores into long streaks. In
addition, the intensity of melanophores can vary
a great deal, with many preserved larvae showing
faint or indistinct melanophore patterns (in some
species this variation is |
|
pronounced, for example in larval Ctenogobius).
Furthermore, melanophores on the delicate membranes
between the fin rays can easily be lost in handling
as the fins get frayed and thus the frequency of
these markings in the few larval types that have
these are undoubtedly higher than observed. Fin
membrane melanophores occur on several common larval
gobies, including Bathygobius
soporator, Coryphopterus
glaucofraenum and Microgobius
signatus, as well as on some eleotrids.
(photographs of melanophore streaks in larval Eleotris
amblyopsis right and contracted vs. expanded
in Bathygobius
mystacium below) |
 |
|
|
|
fin-ray counts
|
| |
| The soft
fin-ray counts often vary by at least one or two
in Caribbean gobies, unlike many other reef fish
families that have very conservative fin-ray formulas.
In addition, the reported modal fin-ray counts from
different sources in the literature can sometimes
vary, usually by one ray. Nevertheless, modal fin-ray
counts are critical to species diagnosis. |
 |
| |
|
| The oft-used
character of six vs. seven spines in the first dorsal
fin is sometimes difficult to see (since the seventh spine
is tiny). Although it is often not that useful for practical
screening of larvae, the number of spines can be very
useful for genus diagnosis, separating genera with otherwise
similar appearances and fin-ray counts. There is some
variation in dorsal spine counts; but it is helpful to
recognize that six-spined gobies usually have five close
spines and then a distant sixth, while seven-spined gobies
have five close spines and then two more spaced out farther.
Thus some of the variants can be recognized as anomalous
(i.e. four close and two spaced out is likely a variant
seven-spined goby). |
| |
| A common problem
is that some literature sources count total dorsal-fin
spines and total dorsal-fin soft-rays, confusing whether
the spine count is including the first, often spinous,
element of the second soft dorsal fin. It is best to count
total elements in the second-dorsal and anal fins to avoid
this problem (and the issue of whether the first element
of the second-dorsal fin and anal fin in some gobies is
spinous or soft, which... surprise, can also vary). |
|
|
Transformation
|
| |
| Larval gobies
tend to initiate transformation from larval to juvenile
phase (also transition, or metamorphosis) while still
pelagic and many transitional individuals can be collected
in waters over the reef. Indeed, in some collections,
the majority of specimens are in transition. As a result,
larval goby samples can often include a surprisingly wide
range of morphological appearances. |
| |
| Head
Shape: The head shape of transitional gobies varies
greatly. Pre-transformation goby larvae usually have thin
pointed heads with terminal mouths. As they initiate transformation,
the head usually thickens and the snout often becomes
more rounded. In those species with blunt head-profiles,
this change can be marked and the mouth can move subterminally.
(photographs below of larval Gobionellus
oceanicus transitional series) |
| |
 |
 |
 |
| |
| Eye
Shape: The eye shape can change radically
during transition and the process is somewhat consistent
within larval types. Both the shape itself and the
size at which the changes are observed can be an
important character for species identification.
As larvae initiate transformation, narrowed eyes
become round, tilted eyes become vertical, and in
some species the eye becomes markedly larger (in
a few it gets smaller, e.g. larval Nes
longus). Eye shapes can thus be valuable
for inferring the stage of goby larvae, i.e. in
a species with narrowed eyes in pre-transitional
larvae, the presence of round eyes in a small individual
indicates that it is in transition. This becomes
particularly useful in practical larval sorting
where the size at which larvae develop round eyes
can be an important character, as in Lythrypnus
vs. Coryphopterus.
(photograph at right of transitional changes in
the eyes of larval Lythrypnus
nesiotes) |
 |
| |
| Body
Shape: The body of pre-transitional larvae
is typically thin and becomes thicker and bulkier
at transition. This change needs to be distinguished
from effects of condition of larvae. Clearly some
emaciated larvae appear very thin and narrow. This
appearance can be found in some larvae with round
eyes and even metamorphic melanophores, indicating
that they are not just immature early-stage larvae. |
| |
|
| The fins of
those species who develop long pectoral and pelvic fins
as juveniles show a marked increase in the length of these
fins at transition. In a few cases, where juveniles have
a characteristically short fin, that fin length may decrease
at transformation. |
| |
| There
is variation in the timing of changes in the early
life history of gobies; some larval gobies develop
transitional morphological changes, especially rounded
eyes and blunted snouts, before acquiring any transitional
markings, as in the larval Lythrypnus
at right. In contrast, it is common with
larval gobies to see individuals of the same species
and in the same collection that have started to
develop metamorphic melanophores while still morphologically
in mid-transition, at least in body and head shape.
However, the eyes of larval gobies almost always
start rounding before transitional markings develop;
it is exceptionally rare to see a larva with dense
metamorphic melanophore patches and narrow eyes.
Two larvae at the ends of the spectrum easily look
like they could be different species. |
 |
 |
|
| |
| Metamorphic
Melanophores: These arrays of additional melanophores
(along with leukophores and iridophores) are usually smaller
and limited to the skin surface, compared to the large,
discrete, and often deeply-penetrating larval melanophores.
In many other reef fish families, the metamorphic melanophores
are typically in dense patches that often begin on the
head and develop posteriorly following the pattern of
the juvenile markings of the species. In gobies, however,
the size difference of the melanophores is less obvious,
and metamorphic melanophores can often be just as large
as larval melanophores and are distinguished mostly by
their graded appearance, i.e. the accumulation of more
markings in a pattern starting around the mouth and head,
then at the caudal peduncle and dorsal midline, and then
filling in from forward to rear (photographs below of
a transitional series of Bathygobius
soporator). This phenomenon helps a great deal
in providing missing links for species IDs, but also contributes
to the confusing variety in the appearance of larval types.
This is especially the case when the metamorphic melanophores
can show up in very different sequences, as is common
in larval Coryphopterus
glaucofraenum. |
| |
|
|
| |
|
Size Variation
|
| |
| Of course
there is some variation in the size of larvae within a
species. There can be two sources of this variation and
distinguishing between them is important. |
| |
| One is the
simple size increase with growth and development during
the early life history: younger and less-developed larvae
are smaller than older ready-to-settle larvae. This variation
can be detected by the well-known ontogenetic landmarks
to be expected with growth, i.e. first the flexion of
the notochord, then the full development of the fin-ray
elements and finally the eye and head shape changes as
settlement approaches. Among the late-stage larvae collected
over reefs, almost all have passed the flexion stage and
have developed their full complement of fin rays. The
subsequent body and eye-shape changes and the degree of
development of metamorphic melanophores are the features
that vary most in these settlement-stage larvae. |
| |
| The
second source of variation is individual variation
in size at the same stage of development. This variation
can be large in gobies, and, of course, the observed
range increases with sample size. This variation
can be confusing, and the occasional extreme size
variant can look like a different species entirely.
For example, the photograph at right shows the extreme
one percent variation in size at transformation
for the common Coryphopterus
glaucofraenum larval type. Note that these
are all transitional larvae that have already developed
round eyes. The larval sizes in the photograph range
from 5.1 to 8.6 mm SL, but 90% of the larvae of
this species that I have collected are concentrated
between 6.5 and 7.3 mm SL. |
 |
|
| |
|
Larval eye morphology
|
| |
| Larval gobies
of different species and different stages of development
exhibit a remarkable variety of shapes of the eyeball,
most often a narrow vertical oval but, in some species,
irregular or even squared. These eye shapes, along with
other eye-related morphological features, likely reflect
adaptations to the pelagic world of reef fish larvae,
either to degrees of darkness or differing wavelengths
of light. Fortunately, these shapes tend to be consistent
within species and can be used as characters to help identify
larvae. |
| |
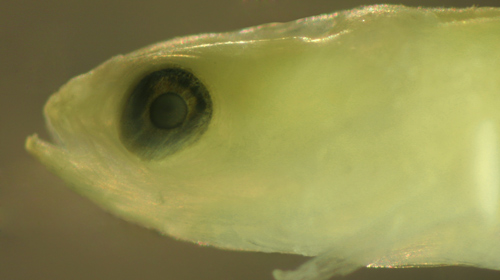 |
| The
primary variations are in eyeball shape, most often
a narrowed vertical oval, but sometimes squared
or another irregular shape. The oval sometimes can
show a pronounced tilt, usually forward, but sometimes
backward. The direction of the tilt is not always
consistent within larval types, for example larval
Evorthodus
lyricus commonly show tilts both forward
and backward (this is true to a lesser degree for
larval parrotfishes, family
Scaridae, as well as the wrasses of family
Labridae). As a rule, the eyes of larval gobies
become fully round at the end of the settlement
transition. |
|
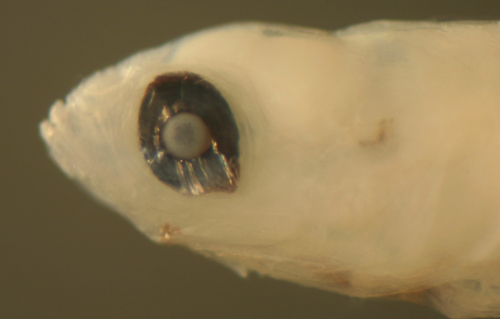 |
|
| In
addition, there can be indentations in the iris,
usually, but not always, dorsal and/or ventral.
Many very early-stage larvae of all kinds of fishes
show these indentations as part of the development
of the eyeball, but in larval gobies these indentations
can persist, sometimes through transition. Persistent
indentations in various quadrants of the iris can
be a consistent character for certain larval types.
The photograph below left shows a persistent dorsal
iris indentation in a 9.6 mm SL transitional larva
of Ctenogobius
saepepallens. The photograph below right
shows a 5.5 mm SL Bathygobius
curacao larva with persistent off-center-axis
dorsal and ventral indentations of the iris despite
being in transition. |
|
|
|
| |
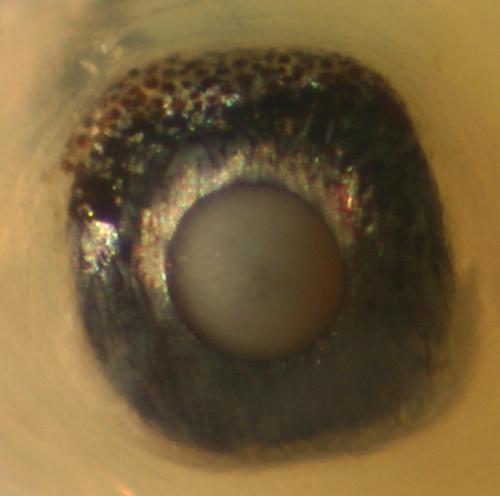 |
| Another
occasional feature of the eyeball of larval gobies
is the presence of an additional speckled membrane
overlying the black surface of the upper iris. This
feature is mostly consistent within larval types
and can thus aid in identifications. In several
larval goby types, this membrane is visibly lifted
off from the eyeball. In some species the speckled
membrane is only along the top quarter of the eyeball,
while in others it extends further down, usually
overlying the posterior half of the iris. At right,
the oblong-shaped eyeball of a 7.2 mm SL larval
Coryphopterus
glaucofraenum shows the distinctly speckled
membrane overlying the upper and rear of the iris. |
|
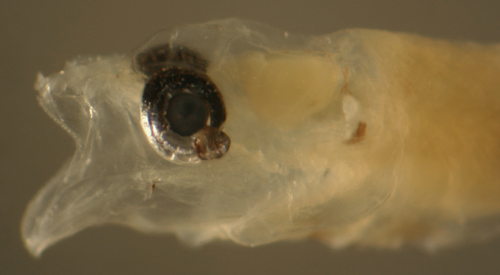 |
|
| A
very common feature in the eyes of larval gobies
is an extension of the shiny iris in the posterior-inferior
quadrant. The extension appears to have a more flattened
appearance than the rest of the iris. In some larval
types this extension is quite prominent. Some rare
individuals show clearly abnormal outgrowths of
the eyeball in this same quadrant, perhaps a developmental
anomaly related to whatever might be the function
of this extension. The photograph at left shows
a 6.9 mm SL larval Coryphopterus
glaucofraenum with the abnormal outgrowth. |
|
| |
| A rare feature
in some larval gobies is a bizarre outgrowth of tissue
from the eyeball into the adjacent compartments of the
head. Interestingly, in
Microgobius signatus this can occur in several
individuals in the same collection, suggesting that whatever
is causing the anomaly may be an environmental effect.
The photograph below shows the head of an 8.0 mm larval
Microgobius signatus. |
| |
|
|
|
|
pelvic-fin morphology
|
| |
 |
| Gobies
are perhaps best known for their fused pelvic
fins that act as a sucking disk to anchor
them to the substrate. The degree of fusion
of the pelvic fins and the overall shape of
the disk are important characters in gobioid
taxonomy, although the feature is certainly
far more labile than taxonomists would desire.
Unfortunately the degree of concordance between
larvae and adults in pelvic-fin morphology
is still an open question. In my collections,
it is clear that the presence or absence of
divided pelvic fins can differ between larval
and adult stages, as in Coryphopterus
personatus. |
| |
|
|
|
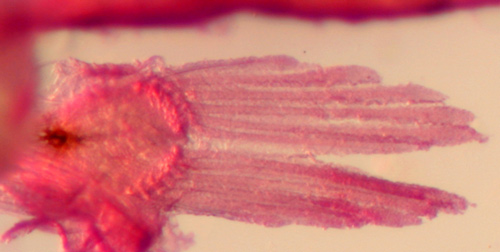
|
|
| There
are several basic states of pelvic-fin morphology
in larval gobies. The pelvic fins on the right and
left can be completely separate, with the base of
the innermost fin ray clearly separated by a space
from the base of the ray on the other side. This
state is typical of most fishes (including the gobioid
sleepers of the family Eleotridae),
but is quite uncommon in gobies. The pelvic fins
can be divided down to the base, or only partially-divided,
leaving the proximal innermost fin rays still fused
(as in larval Gobulus
myersi, pictured at left). Alternatively,
the pelvic fins can be completely fused along the
length of the rays; this is the most |
| common
condition among the larval gobies. Lastly, within
the completely-fused pelvic-fin group, there can
be a frenum, or anterior connecting band, joining
the outermost pelvic-fin spines on the two sides
to form a cup-shaped fin. This cup can be flat and
inconspicuous, as in the 9.9 mm SL larval Ctenogobius
saepepallens at right, or an obvious large
sucking disk as in the 7.7 mm SL larval Elacatinus
saucrus pictured at the top of this section. |
 |
|
| |
|
|
|
 |
|
All contents copyright 2006-2018
All rights reserved
www.coralreeffish.com by
Benjamin Victor
|
|
|
|
|
|