|
|
|
|
Group 4: The long-fin gobies
|
| |
|
Evermannichthys, Evorthodus, Ctenogobius, Gnatholepis, Nes,
Bollmannia, Oxyurichthys,
Gobionellus, Gobioides, Microgobius,
and Palatogobius
(in order of increasing
anal-fin elements)
(Ptereleotris, now assigned to the family Microdesmidae,
closely resembles long-fin gobies as larvae and
is also presented at the bottom of this
page)
|
|
| Gobies
with 12 or more dorsal and/or anal-fin rays
have a generally different look from the short-fin
gobies; they are more likely to have a long
tapering body and a relatively short caudal
peduncle. Although uncommonly encountered
by divers, these gobies are abundant on sand
and silt bottoms near reefs and in brackish
waters along the coast. One species, the Goldspot
Goby Gnatholepis thompsoni, is
commonly seen on the reef. The long-fin gobies
mostly comprise those species that live on
soft substrates, often in holes, and sometimes
with symbiotic shrimp partners. The high number
of fin rays and long narrow bodies are likely
adaptations to hole-dwelling. Their larvae
are typically lightly marked and relatively
small, long, and thin. Those species with
characteristically blunt heads and subterminal
mouths have larvae with pointed snouts and
terminal mouths which undergo marked head-shape
changes at transition. Their larvae can be
very abundant- in the San Blas Islands of
Panama, Ctenogobius saepepallens is the most
frequently collected larval type, occasionally
collected in the thousands at a small nightlight
in a single night. |
|
|
|
| |
|
|
|
|
|
|
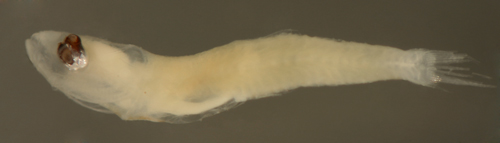
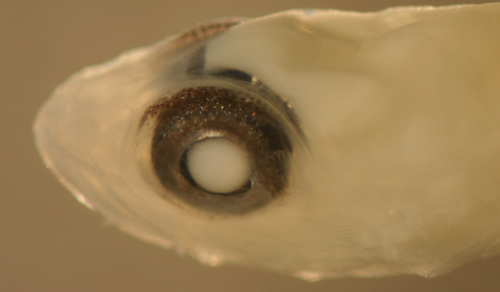
| Evermannichthys metzelaari |
|
|
|
|
|
|
| |
|
|
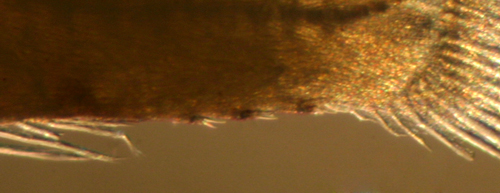
| Diagnosis:
This genus of long eel-like sponge gobies
have normally proportioned larvae. The genus has
notably reduced fin ray numbers: fewer and variable
first-dorsal-fin spine numbers (four to seven),
many fewer pectoral-fin rays (11-13), and only 3-4
procurrent caudal-fin rays. In addition, they have
large spiny scales along the ventral midline of
the caudal peduncle. Fortunately, the characteristic
caudal-peduncle scales are prominent on larvae of
E. metzelaari (Roughtail Goby) and confirms
the identification. Risor
ruber, another sponge goby, also has large
spiny scales along the ventral midline of the caudal
peduncle, but has many more pectoral-fin rays and
only 10 anal-fin elements. There are five poorly
known Caribbean species and the number of fin rays
in this genus varies more than usual, often plus
or minus two rays around the mode: Evermannichthys
metzelaari has a modal fin-ray count of D-IV,15
A-12 Pect-12; E. silus
has D-VI-VII,12-13 A-10-11 Pect-13; E.
convictor has D-V,11-13 A-9-11 Pect-13; E.
spongicola (Gulf of Mexico only?) has D-VI-VII,13
A-10-11 Pect-12; and E.
bicolor has a mode of D-VI,11 A-9. |
|
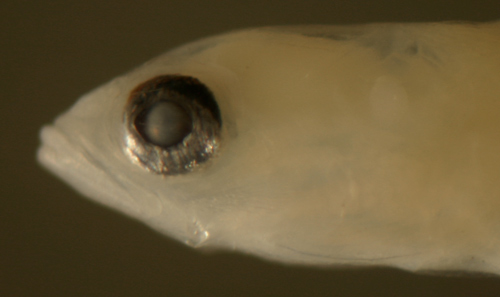
| Description:
Body relatively thin, long, and narrow with a medium
eye, pointed snout, and a large terminal mouth.
Pectoral fins short, pelvic fins long, reaching
almost to the anus. Dorsal and anal-fin bases medium,
caudal peduncle short and narrow with only 3-4 (three
spindly) procurrent caudal-fin rays. Very lightly
marked: internal melanophores at the dorsal surface
of the swim bladder and one melanophore on the caudal
peduncle just after the last anal-fin ray, often
deep and indistinct. There are three or four large
ctenoid scales along the ventral midline of the
caudal peduncle extending from the last anal-fin
ray to the start of the caudal-fin procurrent rays.
Each spiny scale has two large rear-pointing tines
and are outlined in black pigment. Eye shapes range
from a slightly narrowed vertical oval to round.
|
|
|
|
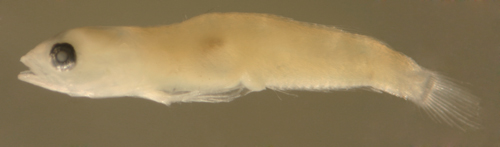
| Evermannichthys
metzelaari larva |
| 6.4 mm SL |
| D-V,15 A-14 |
| San Blas, Panama, SB86-608 |
|
 |
| |
 |
| |
 |
| Evermannichthys
metzelaari larva |
| 6.3 mm SL |
| D-V,15 A-14 |
| San Blas, Panama, SB86-808 |
|
 |
|
|
 |
|
|
|
|
|
|
|
|
|
|
| Diagnosis:
Modal fin-ray counts of D-VI,11 A-12 and Pect-16-17
indicate Evorthodus
lyricus (Lyre Goby), as well as Ctenogobius
boleosoma and C. smaragdus. These
genera typically have one more anal-fin ray than
second-dorsal-fin rays. This larval type has a short
pelvic fin, extending less than half the way to
the anus, separating it from C.
boleosoma. A larval type that is very similar
to this type occurs in the eastern Pacific region
with fin-ray counts matching only to the sibling
species Evorthodus
minutus (also D-VI,11 A-12; there are no
eastern Pacific Ctenogobius
with this fin-ray count). G12 (DNA-confirmed) |
|
| Description:
Body thin, long, and narrow with a small pointed
head, a small to medium-sized eye and a terminal
medium-sized mouth. The widest part of the body
is clearly at the level of the origin of the anal
fin. Pelvic and pectoral fins medium length, reaching
less than half-way to the anus. Dorsal and anal-fin
bases relatively long, caudal peduncle short and
narrowing rapidly, procurrent caudal-fin rays (
spindly). Lightly marked mostly along the lower
body: melanophores along the ventral midline sometimes
at the isthmus, rarely at the pelvic-fin insertion,
and then, most frequent, another just behind the
pelvic-fin insertion. There is sometimes a row of
melanophores along the anal-fin base, variably present
and variably paired (usually only 4 to 7 per side,
can occur on either side unpaired), and sometimes
one or a few extending along the ventral midline
of the caudal peduncle ending before the start of
the procurrent caudal-fin rays. Many individuals,
however, show only indistinct melanophores or no
surface melanophores at all. Melanophores on the
head occur along the upper edge of the anterior
premaxilla (often paired), sometimes also just below
the tip of the dentary and along the sides of the
lower jaw as well. Internal melanophores are present
around the saccule, the dorsal surface of the swim
bladder, and sometimes around the gut near the anus.
Some individuals have a deep melanophore above the
pelvic girdle between the thoracic and abdominal
cavities. Some individuals have paired linear patches
of tiny surface melanophores along the side of the
abdomen just forward of the anus. The eyeball in
this larval type shows an unusual variety of shapes,
with the small eyeball often not round, but irregular
with tilts in all directions, the iris sometimes
off-center, and indentations of the iris in a number
of orientations. Series of transitional larvae show
the eye usually developing from a small slightly
narrowed vertical oval to round and the head shape
changing from a pointed snout to a somewhat blunted
profile. Transitional larvae develop melanophores
in front of the eye and over the upper iris, becoming
a stripe from the eye to the tip of the upper jaw.
In addition, they develop a straight-line bar across
the top of the head behind the eyes, a large melanophore
behind the eye and a row of discrete, often dendritic,
melanophores on the sides of the abdomen just behind
the pelvic-fin insertion. |
|
|
|
| Evorthodus
lyricus larva |
| 8.8 mm SL |
| lightly marked |
| San Blas, Panama, SB84-522 |
|
 |
| |
 |
| |
 |
| Evorthodus
lyricus larva |
| 8.1 mm SL |
| San Blas, Panama, SB84-917 |
|
 |
Evorthodus
lyricus larva (above) vs.
Ctenogobius
boleosoma larva (below) |
| 8.3 and 8.2 mm SL |
| note smaller eye and
shorter pelvic fin |
| San Blas, Panama, SB86-1103 |
|
 |
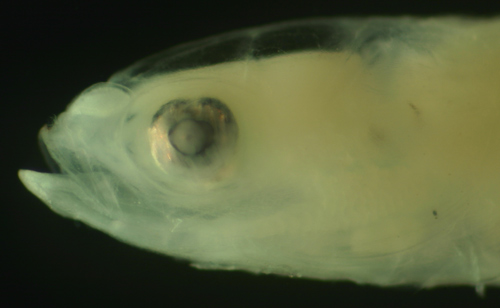
| Evorthodus
lyricus larva |
| 8.3 mm SL |
| small irregular eye |
| San Blas, Panama, SB87-123 |
|
 |
| Evorthodus
lyricus larva |
| 7.9 mm SL |
| patches of tiny melanophores
near anus |
| San Blas, Panama, SB84-522 |
|
 |
| Evorthodus
lyricus larva |
| 8.3 mm SL |
| patches of tiny melanophores
near anus |
| San Blas, Panama, SB86-1001 |
|
 |
| |
 |
Evorthodus
lyricus
early transitional larva |
| 8.4 mm SL |
| San Blas, Panama, SB86-412 |
|
 |
| |
 |
| |
 |
| Evorthodus
lyricus transitional larva |
| 8.5 mm SL |
| San Blas, Panama, SB86-1029 |
|
 |
| |
 |
| |
 |
| Evorthodus
lyricus transitional recruit |
| 10.9 mm SL |
| Colon, Panama, N7529a |
|
 |
|
|
|
|
|
|
|
|
| Diagnosis:
Modal fin-ray counts of D-VI,11 A-12 and Pect-16-17
indicate Ctenogobius
boleosoma (Darter Goby), as well as C. smaragdus,
and Evorthodus
lyricus. These genera typically have one
more anal-fin ray than second-dorsal-fin rays. C.
smaragdus occurs in Florida and Cuba, as well
as Venezuela to Brazil and thus is not included
in this larval type. (DNA-confirmed) |
|
| Analogues:
(post-anal-fin solitary melanophore, large: >9
mm SL) Metamorphic melanophores comprising an
oblique bar forward of the eye to the mid-upper
jaw and pelvic fins almost reaching the anus confirms
C. boleosoma.
A "teardrop" vertical bar from the iris
to the corner of the mouth indicates C.
saepepallens. Evorthodus
lyricus has a markedly shorter pelvic fin
than C. boleosoma
(or C.
saepepallens) and a more appropriate larval
type is identified for that species. Pre-transitional
larvae of this C. boleosoma
type are distinct in having a solitary melanophore
at the tip of the lower jaw, but those without the
spot would be indistinguishable from pre-transitional
C.
saepepallens (since there is some overlap
in fin-ray counts). This conclusion is based on
the fact that larvae in which that is the sole head
marking usually have 12 anal-fin elements and thus
likely represent C.
boleosoma (C.
saepepallens has a mode of 13 anal-fin elements) |
|
| Description:
Body thin, long, and narrow with a large eye and
a terminal medium-sized mouth. Pectoral fins medium
length, reaching about two-thirds of the way to
the anus (longer at transition). Pelvic fins long
and fused with a clear frenum, reaching much of
the way to the anus. Dorsal and anal-fin bases long,
caudal peduncle relatively short and narrow. The
s-shaped gut is usually clearly visible through
the ventral abdominal wall. Lightly marked mostly
along the lower body: melanophores along the ventral
midline at the isthmus (often missing), at the pelvic-fin
insertion, and then sometimes another just behind
the pelvic-fin insertion, after which some individuals
develop a row on each side of the gut strip along
the abdomen. There are several discrete large melanophores
along the anal-fin base, often variably present
and variably paired (usually only 3-4 per side,
but can be up to 7; can occur on either side unpaired),
and a prominent and characteristic melanophore (rarely
two) at the ventral midline of the caudal peduncle
after the last anal-fin ray which has an internal
extension reaching up towards the lateral midline.
In many individuals, especially earlier-stage larvae,
some melanophores are missing or indistinct and
even the characteristic deep peduncle melanophore
is often not visible. Head markings typically develop
as a melanophore between the mid-upper jaw and eye
(any melanophores extending from the eye to the
corner of the mouth or a solitary melanophore on
the iris surface at that quadrant indicate C.
saepepallens; transitional C.
boleosoma do have melanophores on the lower
iris, but always in combination with other transitional
melanophores). Most pre-transitional larvae have
a solitary melanophore at the tip of the lower jaw,
but whether this is a rule is unclear since some
variant transitional larvae recognizable as C.
boleosoma are missing this melanophore. Internal
melanophores are present around the saccule, the
dorsal surface of the swim bladder, and around the
gut near the anus: Some individuals have a deep
melanophore above the pelvic girdle between the
thoracic and abdominal cavities. Series of transitional
larvae show development of the eye from a slightly
narrowed vertical oval to distinctly larger and
round, often with a dorsal dent in the iris that
sometimes persists into the transitional phase.
The head profile develops from a thin pointed head
to a blunt snout with a particularly bulbous head
compared to the midsection. Transitional larvae
develop a pattern of discrete melanophores on the
jaw and the head (primarily in a stripe forward
of the eye and in pairs on top of the head) and
patches of characteristic tiny leukophores (clearly
smaller and more numerous than on C.
saepepallens) on the upper iris, on top
of the head, and in a stripe forward across the
nasal region and along the upper jaw. Patches of
melanophores then develop along the dorsal midline
and on the lateral midline of the caudal peduncle.
Transitional recruits develop additional specklings
of melanophores, leukophores, and iridophores and
a prominent row of lateral midline blotches. A conspicuous
black spot develops at the base of the upper pectoral-fin
rays. |
|
|
| Ctenogobius
boleosoma larva |
| 8.0 mm SL |
| note internal thoracic
melanophore |
| San Blas, Panama, SB86-701 |
|
 |
| |
 |
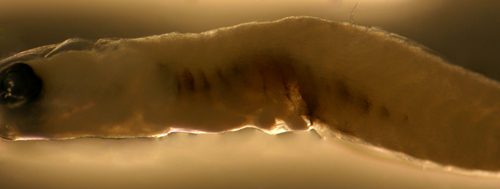
| Ctenogobius?
larva |
| 6.4 mm SL |
| internal melanophore
bars, D-VI,11 A-12 |
| Yucatan, Mexico, 240306 |
| coll. by Lourdes Vasquez
et al. |
|
 |
|
|
 |
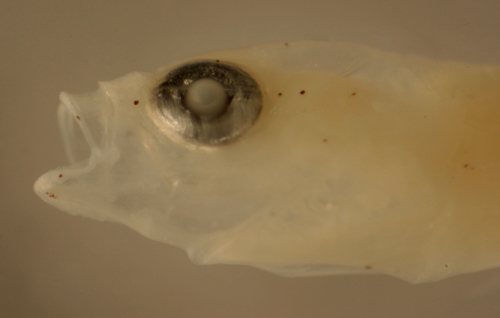
| Ctenogobius
boleosoma larva |
| 8.2 mm SL |
| San Blas, Panama, SB83-156 |
|
 |
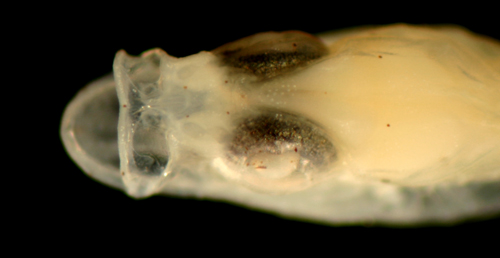
Ctenogobius
boleosoma
early transitional larva |
| 8.9 mm SL |
| melanophore pattern |
| San Blas, Panama, SB83-137 |
|
 |
| |
 |
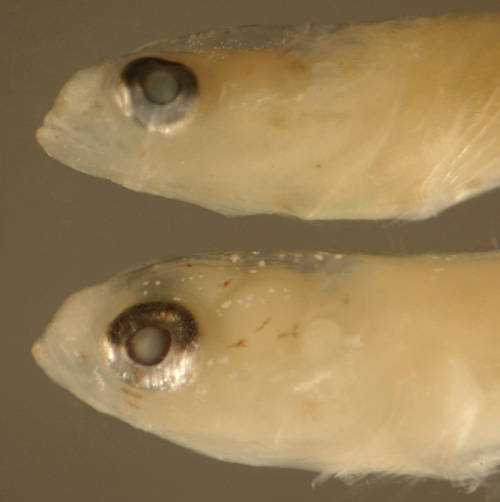
Ctenogobius boleosoma (above) vs.
C.
saepepallens (below) transitional
larvae |
| 8.8 and 8.9 mm SL |
| note leukophore size
differences |
| San Blas, Panama, SB86-809 |
|
 |
| Ctenogobius
boleosoma transitional larva |
| 8.3 mm SL |
| tiny leukophores |
| San Blas, Panama, SB87-218 |
|
 |
| Ctenogobius
boleosoma transitional larvae |
| 8.6 and 8.4 mm SL |
| variant below without
tip-of-lower-jaw spot |
| San Blas, Panama, SB86-1103 |
|
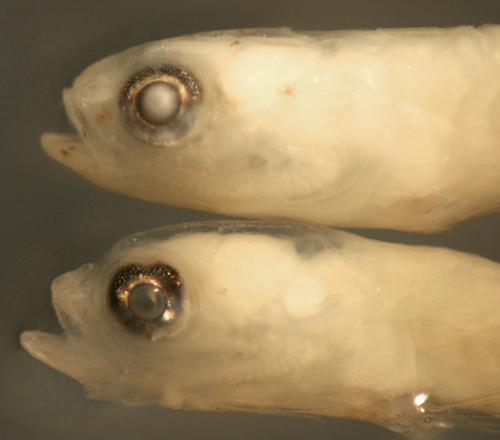 |
Ctenogobius
boleosoma
late transitional larva |
| 9.9 mm SL |
| indistinct leukophores |
| San Blas, Panama, SB86-1002 |
|
 |
Ctenogobius
boleosoma
late transitional larva |
| 9.0 mm SL |
| persistent dorsal iris
indentation |
| San Blas, Panama, SB86-929 |
|
 |
| |
 |
Ctenogobius
boleosoma
late transitional larva |
| 8.8 mm SL |
| San Blas, Panama, SB83-123 |
|
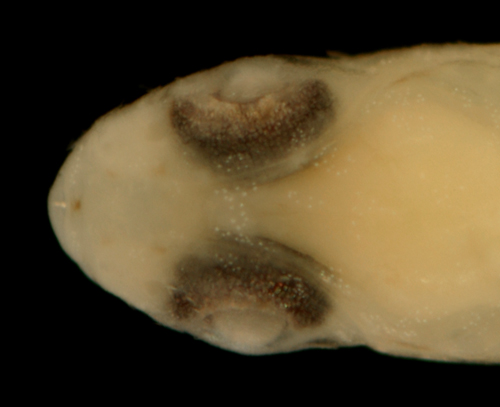 |
| Ctenogobius
boleosoma recruit |
| 9.6 mm SL, pale sand
morph |
| St. Thomas, USVI, ST506 |
|
 |
| |
 |
| Ctenogobius
boleosoma recruit |
| 11.1 mm SL, dark inshore
morph |
| Colon, Panama, N7528b |
|
 |
|
|
 |
|
|
|
|
|
|
|
|
| Diagnosis:
Modal fin-ray counts of D-VI,12 A-13 and Pect-16-17
indicate Ctenogobius
species. These species typically have one
more anal-fin ray than second-dorsal-fin rays. There
are a number of species in the genus that are widespread
and occupy different habitats, and some freshwater
and brackish species with more restricted ranges.
Eight of the ten species share the median-fin ray
count. Ctenogobius
saepepallens (Dash Goby) occurs abundantly
around reefs and in mangroves and is the most common
fish larva collected over reefs in Panama. Turbid-water
species include C.
boleosoma (with modal 11/12), C.
stigmaticus (Pect-18), and C.
stigmaturus (Pect-16). Fresh-water and brackish
species include C.
fasciatus (Pect-17-18), C.
pseudofasciatus, C.
claytonii (Pect-15-17) in the S. Gulf of
Mexico, C. phenacus,
found from Venezuela south, and C.
shufeldti (Pect-17-18) in Florida/Gulf of
Mexico and Venezuela to Brazil. The related
Oxyurichthys stigmalophius has more
fin rays: D-VI,13 A-14 and many more (21-22) pectoral-fin
rays. Vomerogobius
flavus (Lemon Goby) is a rare deeper-water
goby from the Bahamas and Belize/Honduras with similar
fin-ray counts (D-VI,12-13 A-13 Pect-15-16), but
it is very slim, has a distinctly large eye, a wide
gap between the two dorsal fins, and no pelvic frenum:
its larvae are undescribed. (DNA-confirmed) |
| Transitional
larvae and juveniles with a bar from the iris to
the corner of the mouth indicates C.
saepepallens (but the others may not be excluded).
Transitional larvae and juveniles with an oblique
bar forward of the eye to the mid-upper jaw and
not at the corner of the mouth indicate C.
boleosoma. |
| Description:
Body thin, long, and narrow with a large
eye and a terminal medium to small mouth. Pectoral
fins medium length, reaching about two-thirds of
the way to the anus (longer at transition). Pelvic
fins long and fused with a clear frenum, reaching
much of the way to the anus. Dorsal and anal-fin
bases long, caudal peduncle relatively short and
narrow. The s-shaped gut is usually clearly visible
through the ventral abdominal wall. Lightly marked
mostly along the lower body: melanophores along
the ventral midline at the isthmus (often missing),
at the pelvic-fin insertion, and then sometimes
another just behind the pelvic-fin insertion, after
which some individuals develop a row on each side
of the gut strip along the abdomen. There are several
discrete large melanophores along the anal-fin base,
often variably present and variably paired (usually
only 3-4 per side, but can be up to 7; can occur
on either side unpaired), and a prominent and characteristic
melanophore at the ventral midline of the caudal
peduncle after the last anal-fin ray (rarely two)
which has an internal extension reaching up towards
the lateral midline. In many individuals, especially
earlier-stage larvae, some melanophores are missing
or indistinct and even the characteristic peduncle
melanophore is often not visible. The head is unmarked
prior to transition, but many individuals show a
characteristic melanophore on the iris at about
7 o'clock, often with an additional melanophore
extending to the corner of the mouth. Larvae that
have a melanophore just before the tip of the lower
jaw, especially when that is the sole head marking,
usually also have 12 anal-fin elements and are thus
likely C.
boleosoma. However, some transitional larvae
of this C. saepepallens
type have that melanophore, but typically
along with many other head markings. Internal melanophores
are present around the saccule, the dorsal surface
of the swim bladder, and around the gut near the
anus: some individuals have a deep melanophore above
the pelvic girdle between the thoracic and abdominal
cavities. Series of transitional larvae show development
of the eye from a slightly narrowed vertical oval
to distinctly larger and round, often with a dorsal
dent in the iris that sometimes persists into the
transitional phase. The head profile develops from
a thin pointed head to a blunt snout with a particularly
bulbous head compared to the midsection. Transitional
larvae develop a scattering of iridophores and leukophores
on the head along with a pattern of a few discrete
large melanophores on the head behind the eye and
at the base of the pectoral fins. The leukophores
on the top of the head are few, large, and scattered
compared to those on the head of transitional C.
boleosoma larvae. The characteristic bar
below the eye further develops with more melanophores
extending to the corner of the mouth. Melanophores
develop in patches spaced out along the base of
the dorsal fin, at the end of the caudal peduncle
and at the base of the central caudal-fin rays.
Transitional recruits develop additional specklings
of melanophores and leukophores and a lateral midline
row of melanophore patches. A black spot develops
at the base of the upper pectoral-fin rays. |
|
|
|
| Ctenogobius
saepepallens larva |
| 10.1 mm SL |
| San Blas, Panama, SB86-921 |
| |
|
 |
| Ctenogobius
saepepallens larva |
| 10.0 mm SL |
| San Blas, Panama, SB86-627 |
|
 |
| Ctenogobius
saepepallens larva |
| 9.2 mm SL |
| San Blas, Panama, SB86-815 |
|
 |
| Ctenogobius
saepepallens larvae |
| 8.1 to 9.5 mm SL |
head shape and eye
development,
melanophore variation |
| San Blas, Panama, SB83-151 |
|
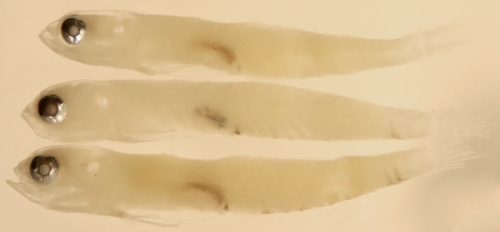 |
| Ctenogobius
saepepallens larva |
| 9.9 mm SL |
| pelvic frenum, abdominal
melanophores |
| San Blas, Panama, SB86-623 |
|
 |
| |
 |
| Ctenogobius
saepepallens larvae |
| 9.2 and 9.3 mm SL |
| S-shaped gut, melanophore
variation |
| San Blas, Panama, SB86-623 |
|
 |
| Ctenogobius
saepepallens + larva |
| 6.7 mm SL |
| small but not early
stage, eye is round |
| San Blas, Panama, SB86-502 |
|
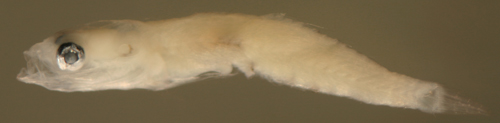 |
| |
 |
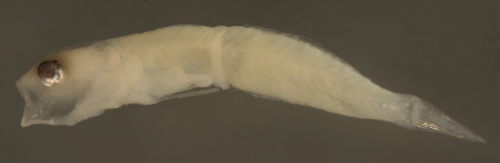
| Ctenogobius
saepepallens + larva |
| 7.8 mm SL |
| small with indistinct
melanophores |
| San Blas, Panama, SB84-529a |
|
 |
| |
 |
| Ctenogobius
saepepallens larva |
| 9.1 mm SL |
| melanophores indistinct
or absent |
| San Blas, Panama, SB84-520 |
|
 |
| |
 |
Ctenogobius
saepepallens
early transitional larva |
| 9.9 mm SL |
| deep caudal peduncle
melanophore |
| San Blas, Panama, SB86-409 |
|
 |
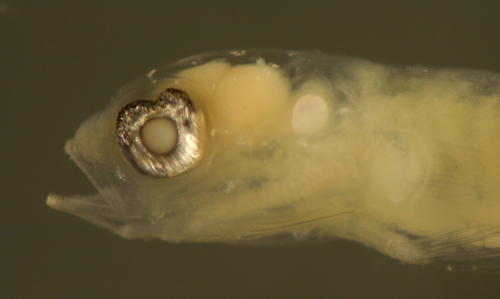
Ctenogobius
saepepallens
early transitional larva |
| 9.6 mm SL |
| leukophores and iridophores
only |
| San Blas, Panama, SB84-624a |
|
 |
Ctenogobius
saepepallens
transitional larva |
| 9.0 mm SL |
| San Blas, Panama, SB84-405 |
|
 |
| |
 |
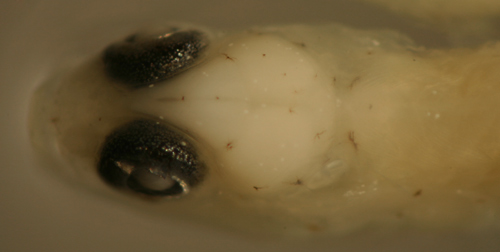
Ctenogobius
saepepallens
transitional larva |
| 9.5 mm SL |
| San Blas, Panama, SB87-218 |
|
 |
| |
 |
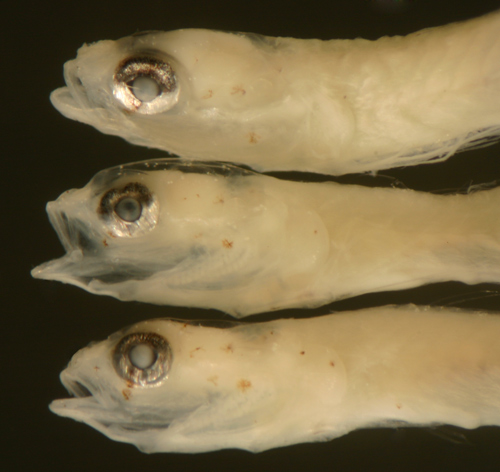
Ctenogobius
saepepallens
transitional larvae |
| 9.3, 9.3, and 9.0 mm
SL |
| head melanophore variation |
| San Blas, Panama, SB86-405 |
|
 |
| |
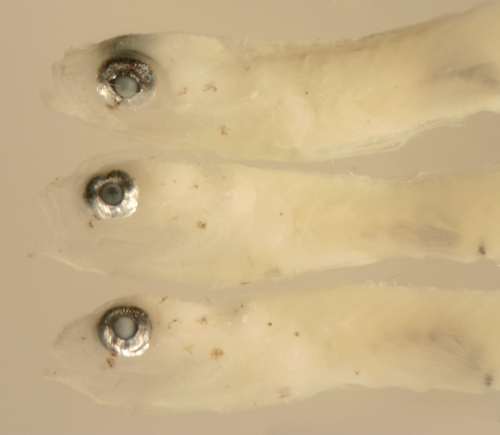 |
Ctenogobius
saepepallens
early transitional larvae |
| 8.7 and 9.7 mm SL |
| eye changes |
| San Blas, Panama, SB87-218 |
|
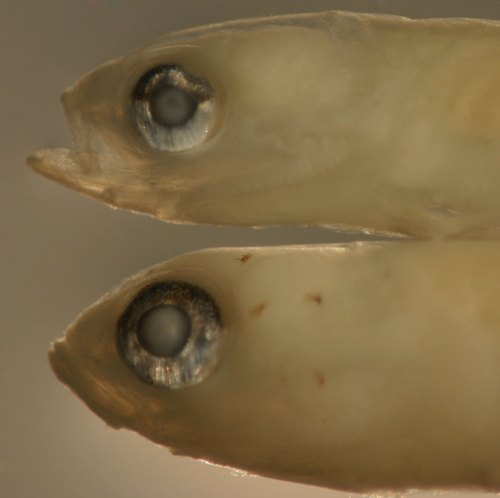 |
Ctenogobius
saepepallens
transitional recruit |
| 9.4 mm SL |
| San Blas, Panama, SB82-097 |
|
 |
| |
 |
| Ctenogobius
saepepallens juvenile |
| 15.7 mm SL |
| San Blas, Panama, SB83-111 |
|
 |
|
|
|
|
|
|
|
|
| Diagnosis:
Modal fin-ray counts of D-VI,12 A-12 Pect-17 indicate
Gnatholepis thompsoni
(Goldspot Goby). (DNA-confirmed) |
|
| Analogues:
(long, and narrow, no anal-fin base row of melanophores)
Similar median-fin ray counts occur in Bollmannia
litura, but that genus has seven first-dorsal-fin
spines and many more pectoral-fin rays (20 or more).
Rare individuals of Ctenogobius
saepepallens , and probably others of that
genus, can have equal numbers of anal and second-dorsal-fin
ray elements and thus match the counts of this larval
type. Most long, and narrow larval gobies have a
row of melanophores along the anal-fin base, but
among some groups there are often individuals without
markings (primarily Ctenogobius
spp. and Evorthodus
lyricus). Those individuals can resemble
larval Gnatholepis thompsoni, but usually
have one more anal-fin element than second-dorsal-fin
elements vs. equal numbers in Gnatholepis thompsoni
larvae. In addition, larval Gnatholepis thompsoni
have a distinctly larger eye, a smaller downturned
mouth, and melanophores along the dorsal fins. The
transitional melanophore patterns are also completely
different. Larval Evermannichthys
spp. have a larger mouth, a sharply-pointed
snout, and spiny caudal peduncle scales. |
|
| Description:
Body thin, long, and narrow with a large eye and
relatively small somewhat downturned mouth. Pectoral
fins medium length, pelvic fins medium length and
fused with a clear frenum, dorsal and anal-fin bases
long, caudal peduncle relatively long and somewhat
narrow. Pretransitional larvae have melanophores
only on the dorsal fins, along the membranes between
the dorsal-fin spines and the first three or four
second-dorsal-fin elements (these can be missing
on larvae with frayed fins). Series of transitional
larvae show the eye remaining large and round, often
with a prominent dorsal, occasionally a ventral,
indentation in the iris. Transitional larvae develop
a melanophore on the proximal middle pectoral-fin
rays and rows of large melanophores on the body
near the base of the dorsal fins. The characteristic
comma-shaped bar below the eye then develops from
the iris down and a corresponding bar develops on
the upper iris onto the dorsal surface of the head.
Leukophore patches form over the top of the head
and over the iris, on the cheek, and on the base
of the pectoral fin. |
|
|
|
| Gnatholepis
thompsoni transitional larva |
| 9.9 mm SL |
| San Blas, Panama, SB84-624a |
| |
|
 |
| Gnatholepis
thompsoni transitional recruit |
| 10.3 mm SL |
| Colon, Panama, N7529b |
| |
|
 |
| |
 |
|
|
|
|
|
|
|
|
| Diagnosis:
Modal fin-ray counts of D-VII,13-14 A-13 (11-13)
Pect-17 indicate Nes longus (Orangespotted
Goby), as well as the deeper water Parrella
macropteryx (Longtail Goby). The latter species
is apparently rare and poorly documented from only
a few scattered locations in the Caribbean, typically
trawled from deep soft-bottom habitat; its larvae
are unknown. (DNA-confirmed) |
|
| Analogues:
(post-anal-fin solitary melanophore, large: >9
mm SL) Within the diverse solitary post-anal-fin
melanophore group, there are few species with large
larvae: the only other large larva comparable to
Nes longus is that of Psilotris
amblyrhynchus. The similarity is especially
notable when larval Nes longus are missing
their anterior anal-fin base melanophores as well
as their caudal-fin base melanophores, which is
not uncommon. In that case, P.
amblyrhynchus larvae can be separated by
pelvic fin morphology (mostly divided pelvic fins
with no frenum vs. fused and an obvious frenum in
Nes longus)
and fewer median-fin rays (usually 12/11 vs. 13-14/11-13).
Other similar goby larvae are much smaller: some
with divided pelvic fins and typically other distinctive
melanophores (Psilotris,
Gobulus
myersi, and Pycnomma
roosevelti) and, with fused pelvic fins
(and usually only the solitary post-anal-fin melanophore),
some Gobiosoma
and Elacatinus,
as well as Evermannichthys
(the latter also with a sharply-pointed snout and
spiny caudal peduncle scales). |
|
| Description:
Body somewhat thick, long, and narrow with a large
head and medium-sized eye and terminal large mouth.
Pectoral fins medium length, not reaching to the
level of the anus, pelvic fins medium length as
well and fused with a clear frenum. Dorsal and anal-fin
bases medium length, caudal peduncle relatively
long, and narrowing rapidly. Lightly marked along
the lower body: melanophores limited to a streak
in front of the pelvic-fin insertion (often obscure,
sometimes absent), a melanophore or two per side
(variably paired) at the base of the anterior anal-fin
rays (sometimes absent, occasionally one more at
the mid-anal fin as well), and a large prominent
dendritic melanophore at the ventral midline after
the last anal-fin ray (often spreading over the
base of the last anal-fin rays). Internal melanophores
are present above the the rear brain case at the
midline, single and often obscured, along the dorsal
surface of the swim bladder and around the gut near
the anus (often dendritic and extending down to
the surface around the anus). Many individuals also
have one or two melanophores at the base of one
or two of the lower segmented caudal-fin rays. Series
of transitional larvae show development of the eye
from a slightly narrowed vertical oval to round
and then, particularly unusual for gobies, becoming
relatively much smaller as the head shape changes.
The head widens and broadens markedly as the body
stays relatively narrow and the pelvic fins become
shorter. Transitional larvae develop melanophores
in an arc behind the upper eye, between the eye
and the mid-upper jaw, at the angle of the jaw and
below the mid-dentary of the lower jaw. Transitional
larvae have a bubblewrap-like skin. |
|
|
|
| Nes
longus larva |
| 10.7 mm SL |
| Carrie Bow Cay, Belize
1986 |
|
 |
| |
 |
| Nes
longus larvae |
| both 9.2 mm SL |
| note internal head
melanophore |
| San Blas, Panama, SB87-225 |
|
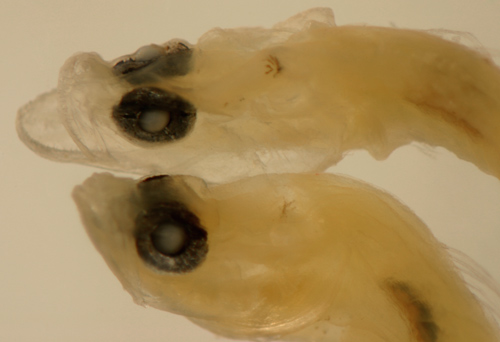 |
| Nes
longus early transitional larva |
| 9.9 mm SL |
| shorter pelvic fins |
| San Blas, Panama, SB86-929 |
|
 |
| |
 |
| |
 |
| |
 |
| Nes
longus early transitional larva |
| 9.7 mm SL |
| San Blas, Panama, SB81-047 |
|
 |
| Nes
longus early transitional larva |
| 9.8 mm SL |
| head melanophores,
bubblewrap-like skin |
| San Blas, Panama, SB87-225 |
|
 |
| |
 |
| |
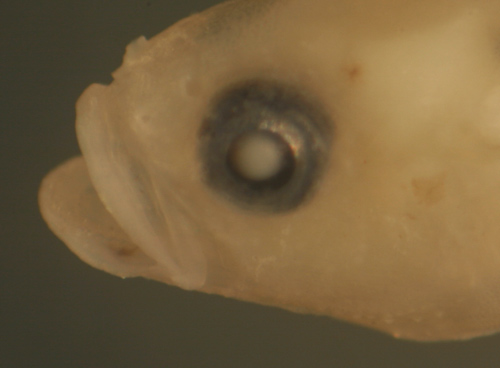 |
| Nes
longus transitional larva |
| 9.1 mm SL |
transitional morphology
only
head broadens, eye much smaller |
| San Blas, Panama, SB83-163 |
|
 |
| |
 |
| Nes
longus transitional larva |
| 9.0 mm SL |
| San Blas, Panama, SB86-1120 |
|
 |
|
|
|
|
|
|
|
|
| Diagnosis:
Modal fin-ray counts of D-VII,13 A-13 and
Pect-20-22 indicate Bollmannia
boqueronensis (White-eye Goby). Second-dorsal-fin
elements 12-15 and anal-fin elements 12-14 with
equal numbers of elements and high pectoral-fin-ray
counts over 20 are only found in
Bollmannia (Gobionellus,
Gobioides,
and Microgobius
all have more than 15 anal-fin elements, Parrella
macropteryx has only 17 pectoral-fin rays).
B. boqueronensis
has a mode of 13 second dorsal and anal-fin elements
and 20-21 pectoral-fin rays. Other regional species
comprise B. litura
with 12/12 and 20 pectoral-fin rays, B.
eigenmanni with 12/13 and 23-25 pectoral-fin
rays, and B. communis
(USA to Campeche) with a mode of 15/14 and 22 pectoral-fin
rays. Some other gobies overlap the median-fin soft
ray counts, but all have six first-dorsal-fin spines:
Oxyurichthys stigmalophius with one
more anal than dorsal-fin rays (D-13 A-14, Pect-21-22),
a few Ctenogobius
species (but they have many fewer pectoral-fin rays),
and Vomerogobius flavus
(D-VI,12-13 A-13, but Pect-15-16). (U) |
|
| Note:
Earlier-stage larvae are somewhat different
in appearance and it is not certain that these types
all represent the same species. The fin-ray count
is somewhat distinctive and all specimens share
the count. A 7.1 mm SL larva with stubs of pelvic
fins and melanophores in a row along the ventral
midline of the abdomen and on the dorsal midline
of the caudal peduncle resembles a larvae identified
as Bollmannia communis
by Ruple (2004; via Richards' (2006) goby chapter),
but my larva has melanophores at the base of the
lower caudal-fin rays not illustrated by Ruple and
one fewer dorsal and anal-fin rays. Larger and transitional
specimens, over 9.0 mm SL, are missing the midline
abdominal melanophores, but share with the 7.1 mm
SL specimen the fin-ray count, the melanophore at
the angle of the jaw, the speckled membrane above
the upper eyeball, the caudal peduncle melanophore
(sometimes) and the large slightly underslung mouth,
making it likely that they represent a single series
of Bollmannia sp.
The early-stage 5.9 mm SL larva shares the fin-ray
count and the unusual irregular eye shape with the
7.1 mm SL specimen. |
|
| Description:
Body thin, long, and narrow with a large eye and
mouth. Pectoral fins long, pelvic fins long (in
larvae above 9 mm SL, stubs in 7.1 mm SL and less),
dorsal and anal-fin bases medium length, caudal
peduncle long, and narrowing rapidly, 8-9 procurrent
caudal-fin rays (8 spindly). Lightly marked mostly
along the ventral midline: melanophores in streaks
at the posterior isthmus and along the pelvic-fin
insertion, then four midline melanophores along
the abdomen (only in the 7.1 mm SL larva), the last
just forward of the anus, then a row along the anal-fin
base behind the second anal-fin element (variably
paired, one per side) and then a long streak extending
along the ventral midline of the caudal peduncle
ending near the start of the procurrent caudal-fin
rays. There are melanophores at the base of some
of the lower segmented caudal-fin rays. The only
dorsal marking is a melanophore on the dorsal midline
of the caudal peduncle some distance after the last
dorsal-fin ray (missing in some individuals). There
are melanophores at the angle of the jaw in the
larvae over 7.0 mm SL. There are internal melanophores
along the dorsal surface of the swim bladder and
around the gut near the anus. The eye is unusual,
ranging from a large irregular oblong tilted forward
with a ventral anterior indentation in the iris
and a posterior-inferior iris extension in earlier-stage
larvae becoming a moderately-narrowed vertical oval
in the largest larvae. Larvae over 7 mm SL also
have a speckled "eyebrow" membrane over the upper
third of the eyeball that appears detached from
the pigmented iris below. |
|
|
|
| Bollmannia
boqueronensis ? larva |
| 5.9 mm SL |
| earlier stage larva |
| San Blas, Panama, SB84-522 |
|
 |
| |
 |
| Bollmannia
boqueronensis larva |
| 7.1 mm SL |
second dorsal with
12, A-13 elements
pectoral fin incomplete but >20 |
| San Blas, Panama, SB86-1006 |
|
 |
| |
 |
| |
 |
| |
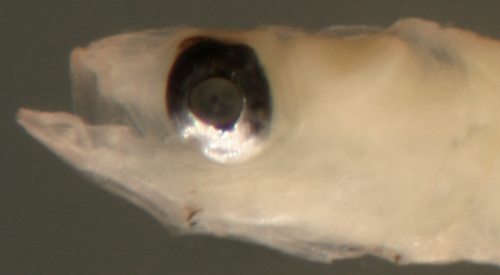 |
| Bollmannia
boqueronensis larva |
| 9.3 mm SL |
| San Blas, Panama, SB82-044 |
|
 |
| |
 |
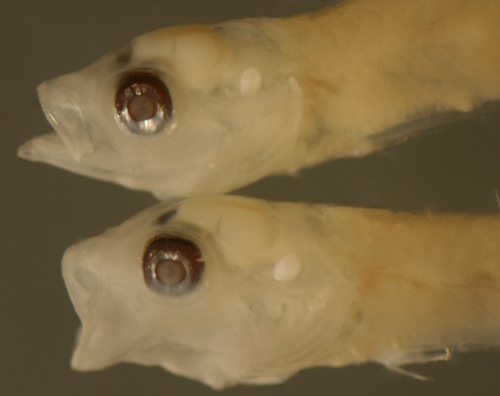
| Bollmannia
boqueronensis larvae |
| 9.3 and 9.1 mm SL |
| San Blas, Panama, SB82-044 |
|
 |
| Bollmannia
boqueronensis larva |
| 9.1 mm SL |
| note speckled eyebrow
membrane |
| San Blas, Panama, SB82-044 |
|
 |
| Bollmannia
boqueronensis larva |
| 9.4 mm SL |
| note dorsal caudal
peduncle spot |
| San Blas, Panama, SB81-001 |
|
 |
|
|
|
|
|
|
|
|
| Diagnosis:
Modal fin-ray counts of D-VI,14 A-15 and
Pect-19 indicate Gobionellus
oceanicus (Highfin Goby). This genus typically
has one more anal-fin ray than second-dorsal-fin
rays (sometimes equal). A number of other species
have spent some time in this genus, but Pezold (2004)
recognizes only the one Caribbean species. (U) G9
|
|
| Description:
Body thin, very long, and narrow with a small eye
and a pointed snout with a terminal large mouth.
Pectoral and pelvic fins long relative to the head
(but short compared to the long body), extending
more than halfway to the anus. Dorsal and anal-fin
bases very long, caudal peduncle short, . Lightly
marked along the lower body: melanophores usually
in streaks at the isthmus (often missing) and at
the pelvic-fin insertion, internally at the dorsal
surface of the swim bladder and around the gut near
the anus, and in a row along the anal-fin base,
often variably present and variably paired (can
occur on either side unpaired). In many individuals
the surface melanophores are indistinct or some
are missing. Series of transitional larvae show
development of the eye from a markedly narrowed
vertical oval with a flattened base, the pupil off-center
dorsally, and a pronounced slant backwards to large
and round. The head profile develops from a thin
pointed head to a blunt snout with an almost sub-terminal
mouth. Transitional larvae first develop patches
of tiny iridophores on the top of the head and in
a stripe behind the eye and then a scattering of
large discrete melanophores on the head. Body markings
include a lateral row of melanophores on each side
of the gut strip along the abdomen. Melanophores
develop in patches spaced out along the base of
the dorsal fin, on the caudal peduncle, and at the
base of the central caudal-fin rays. |
|
|
|
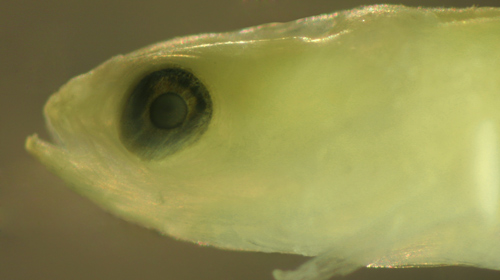
| Gobionellus
oceanicus larva |
| 12.5 mm SL |
| note eye tilted sharply
backward |
| San Blas, Panama, SB81-002 |
|
 |
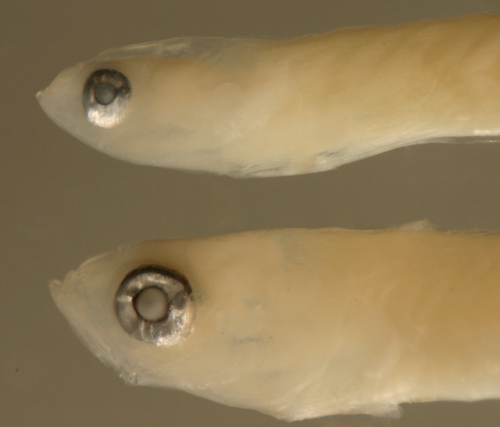
Gobionellus
oceanicus larva above
vs. Microgobius
signatus below |
| 13.4 mm SL |
| San Blas, Panama, SB86-528 |
|
 |
Gobionellus
oceanicus
early transitional larva |
| 13.3 mm SL |
| San Blas, Panama, SB86-728 |
|
 |
 |
| |
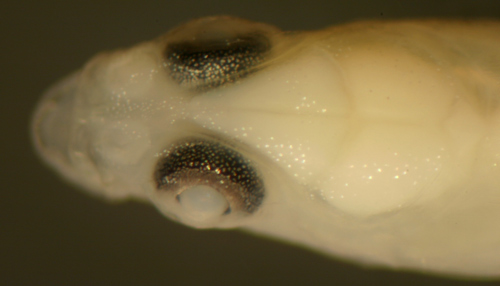 |
| Gobionellus
oceanicus transitional larva |
| 13.4 mm SL |
| San Blas, Panama, SB86-405 |
|
 |
| |
 |
| Gobionellus
oceanicus transitional larva |
| 12.9 mm SL |
| San Blas, Panama, SB86-1103 |
|
 |
| |
 |
| |
 |
| Gobionellus
oceanicus transitional larvae |
| 12.7 (above) and 12.9
mm SL |
| San Blas, Panama, SB86-1103 |
|
 |
| Gobionellus
oceanicus transitional larva |
| 12.8 mm SL |
| San Blas, Panama, SB86-929 |
|
 |
| |
 |
|
|
|
|
| |
| Oxyurichthys stigmalophius |
|
|
|
|
|
|
|
|
|
| Diagnosis:
Modal fin-ray counts of D-VI,13 A-14 and
Pect-21-22 and separated dorsal fins indicates Oxyurichthys
stigmalophius (Spotfin Goby). This genus, with
only a single Atlantic species, typically has one
more anal-fin ray than second-dorsal-fin rays. No
larvae have been reported in the literature or collected
by scientists. The only record is a few photographs
of a larva about 20-25 mm long, taken at night over
sand at 6 feet deep in Bonaire by Chris Spray: the
photographs are so detailed that they are by far
the best documentation of a larva one could expect. |
|
|
Description: Body
thin, long, and narrow with a moderately large
round eye and a short snout with a terminal large
mouth. Pectoral and pelvic fins relatively short.
Dorsal and anal-fin bases long, caudal peduncle
short. Relatively heavily marked: melanophores
on both head and body, mainly along ventral midline
as (probably) streaks at the isthmus and at the
pelvic-fin insertion, followed by a row of prominent
large melanophores along the base of the anal
fin at each soft ray and continuing along the
ventral midline of the caudal peduncle. Internally,
melanophores concentrate over the dorsal surface
of the swim bladder, notably continuing in a curve
around the posterior margin of the swim bladder.
Apparently a row of 5 or 6 small internal melanophores
above the spine, spaced evenly along body, ending
with a melanophore at midline near the end of
the caudal peduncle; a similar series of streak
melanophores along the ribs, at least anteriorly.
Head with many scattered melanophores, concentrated
along the upper lip, lower jaw, snout in front
of pupil, and several large melanophores over
the cranium and a few more smaller melanophores
just posterior to the cranium and perhaps covering
the otoliths. Fins mostly unmarked, except for
the caudal fin, with melanophores at the base
of the upper as well as the lower rays and a few
scattered melanophores extending along the mid
and lower ray shafts.
Erythrophores scattered mostly along upper body,
in a series of 5 or 6 spots spaced along the dorsal
midline, a similar series of streaks along myomere
margins, and a similar series along the lateral
midline, and overlying the dark spot on the midline
caudal-peduncle and in a vertical line along the
base of the caudal fin and scattered along the
shafts of the lower caudal-fin rays.
|
|
|
|
| Oxyurichthys stigmalophius
settling larva |
| approx. 20 mm SL |
| Bonaire, courtesy Chris
Spray |
|
 |
|
|
 |
| |
 |
|
|
|
|
| |
|
|
|
| Diagnosis:
Modal fin-ray counts of D-VII (last two longest),15-16
A-16-17 and Pect-18-19 and continuous dorsal fins,
small eye, and short pectoral and pelvic fins indicate
Gobioides broussonnetii (Violet Goby). The
species name is frequently misspelled as broussonetii
or broussoneti. G. grahamae occurs in Guyana
south to Brazil. Akko dionaea, an obscure
mud-bottom brackish-water goby found at the mouth
of large rivers in Colombia and Brazil, has D-VII,15
A-15 Pect-16-18. |
|
|
|
|
|
|
|
|
|
|
|
|
|
| Diagnosis:
Modal fin-ray counts of D-VII,16-17 (15-18)
A-16-17 (16-19) and Pect-20-24 are shared by several
Microgobius species: M.
carri (Seminole Goby) (widespread in the
Caribbean) and M. meeki
from Puerto Rico and the southern Caribbean, as
well as M. gulosus
(D-VII,15-17 A-16-18 and Pect-20-23) and M.
thalassinus (D-VII,15-17 A-16-17 and Pect-20-22),
both found in temperate US waters and the Gulf of
Mexico only. This genus typically has one more anal-fin
ray than dorsal-fin rays (but often equal numbers).
(ML) |
|
| Description:
Body thin, long, and narrow with a large eye and
a terminal mouth. Pectoral fins short, pelvic fins
stubs until transition, dorsal and anal-fin bases
very long, caudal peduncle very short and narrow,
7-9 procurrent caudal-fin rays (7-8 spindly). Lightly
marked along the lower body: melanophores usually
in long streaks at the posterior isthmus, at the
pelvic-fin insertion and then continuing as an abdominal
midline streak ending about half way between the
pelvic-fin insertion and the swim bladder (vs. eleotrids
where it extends to the swim bladder). There is
a row of melanophores along the anal-fin base (variably
paired, one per side, a few larger ones starting
at the second or third element that are fewer than
one per ray, then becoming small and usually one
per ray), and then a streak along the ventral midline
of the very short caudal peduncle ending at the
start of the procurrent caudal-fin rays. Melanophores
are present on some of the anal-fin ray membranes,
usually between the first six elements near the
base of the ray (the frequency of occurrence of
the anal-fin ray membrane melanophores is uncertain
since many larvae have frayed fin rays). Melanophores
are present at the base of most of the lower segmented
caudal-fin rays extending a short way out along
the rays; in larger individuals there are melanophores
at the base of the central and some of the upper
segmented caudal-fin rays as well. Some larger larvae
have melanophores at the angle of the jaw and around
the sacculus, but on many larvae these are absent.
Internal melanophores are present at the dorsal
surface of the swim bladder and often around the
gut near the anus. Size series of larvae show variable
eye shapes: at around 6 mm SL the eye is round with
dorsal and ventral indentations in the iris and
a posterior-inferior exension of the iris, then
between 7 and 11 mm SL the eye is a somewhat-narrowed
vertical oval, often tilting slightly forward, with
a prominent posterior-inferior extension of the
iris. At transitional sizes (11-15 mm SL), larvae
develop large round eyes. Many larvae have a speckled
"eyebrow" membrane over the upper third of the eyeball
that appears detached from the pigmented iris below.
Transitional larvae develop internal melanophores
within the caudal peduncle and the pelvic fins extend
in length rapidly. A melanophore appears behind
the upper edge of the operculum above a streak of
iridophores. Transitional recruits develop a stripe
from the eye to the caudal peduncle and speckling
along the bases of the median-fin rays and a row
of melanophores along the lateral wall of the abdomen
below the pectoral fin. |
|
|
|
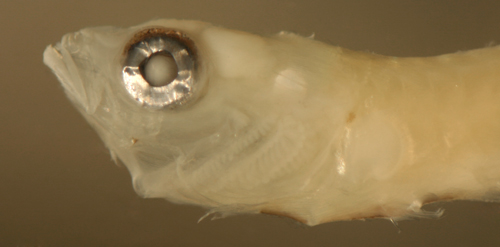
| Microgobius
transitional larva |
| 12.3 mm SL |
| San Blas, Panama, SB81-019 |
|
 |
| Microgobius
carri transitional larva |
| 13.2 mm SL |
| San Blas, Panama, SB86-1120 |
|
 |
| |
 |
| Microgobius
carri transitional recruit |
| 14.9 mm SL |
| San Blas, Panama, SB82-033 |
|
 |
| |
 |
|
|
|
|
|
|
|
|
| Diagnosis:
Modal fin-ray counts of D-VII,16-17 (15-18)
A-16-17 (16-19) and Pect-21-22 are shared by several
Microgobius species: M.
carri (widespread in the Caribbean), M.
meeki from Puerto Rico and the southern Caribbean,
as well as M. gulosus
(D-VII,15-17 A-16-18 and Pect-20-23) and M.
thalassinus (D-VII,15-17 A-16-17 and Pect-20-22),
both found in temperate US waters and the Gulf of
Mexico only. This genus typically has one more anal-fin
ray than dorsal-fin rays (but often equal numbers).
(ML) |
|
| Description:
. |
|
|
|
|
|
|
|
|
|
|
| Diagnosis:
Modal fin-ray counts of D-VII,18-19 (16-20)
A-19 (18-20) Pect-21-22 indicate Microgobius
microlepis (Banner Goby); found in Florida
and down to Belize, in the NW Caribbean region.
This genus typically has one more anal-fin ray than
dorsal-fin rays (but often equal numbers). (PE)
G318/19 |
|
|
|
|
|
|
|
|
|
|
|
|
|
| Diagnosis:
Modal fin-ray counts of D-VII,20 (19-22)
A-21 (20-22) Pect-19-22 indicates Microgobius
signatus (Signal Goby), and the counts overlap
Palatogobius paradoxus. This larval type
is quite common, inconsistent with the latter relatively
rare and deeper-water species. Microgobius
typically have one more anal-fin ray than dorsal-fin
rays (but often equal numbers, rarely one more dorsal
ray). Ptereleotris
helenae (previously Ioglossus helenae)
has 22 or more second-dorsal-fin elements (along
with only six first-dorsal-fin spines and separate
pelvic fins). (U) |
|
| Description:
Body thin, long, and narrow with a large eye and
a large terminal mouth. Pectoral fins short, pelvic
fins stubs, dorsal and anal-fin bases very long,
caudal peduncle very short and narrow, procurrent
caudal-fin rays 7-9 (7-8 spindly). Lightly marked
along the lower body: melanophores usually in long
streaks at the posterior isthmus, at the pelvic-fin
insertion and then continuing as an abdominal midline
streak ending about half way between the pelvic-fin
insertion and the swim bladder (vs. eleotrids where
it extends to the swim bladder). There is a row
of melanophores along the anal-fin base (variably
paired, one per side, a few larger ones starting
at the second or third element that are fewer than
one per ray, then becoming small and usually one
per ray), and then a streak along the ventral midline
of the very short caudal peduncle ending at the
start of the procurrent caudal-fin rays. Melanophores
are present on some of the anal-fin ray membranes,
usually between the second and sixth elements, and
along the base of some of the lower segmented caudal-fin
rays extending a short way out along the rays. The
frequency of occurrence of the anal-fin ray membrane
melanophores is uncertain, since many larvae have
frayed fin rays. Some larger larvae have melanophores
at the angle of the jaw and around the sacculus,
but on many larvae, especially smaller specimens,
these are absent. Internal melanophores are present
at the dorsal surface of the swim bladder and often
around the gut near the anus. Size series of larvae
show variable eye shapes at early stages: at around
6 mm SL the eye is round with dorsal and ventral
indentations in the iris and a posterior-inferior
exension of the iris, then between 7 and 11 mm SL
the eye is a somewhat-narrowed vertical oval, often
tilting slightly forward, with a prominent posterior-inferior
extension of the iris. Some larvae at this stage
have unusual outgrowths, extensions, and distortions
of the eyeball. At transitional sizes (11-13 mm
SL), larvae develop large round eyes. Many larvae
have a speckled "eyebrow" membrane over the upper
edge of the eyeball that appears detached from the
pigmented iris below. The iris in this genus has
a subtle, yet distinctive, "flat" appearance with
a more uniform shine than is present on the eyes
of other goby larvae. |
|
|
|
|
|
 |
| Microgobius
sp.larva |
| 6.6 mm SL |
| earlier stage larva |
| San Blas, Panama, SB86-502 |
|
 |
| |
 |
| Microgobius
sp. ? larva |
| 6.6 mm SL |
| thin variant, with
round eye |
| San Blas, Panama, SB84-522 |
|
 |
| |
 |
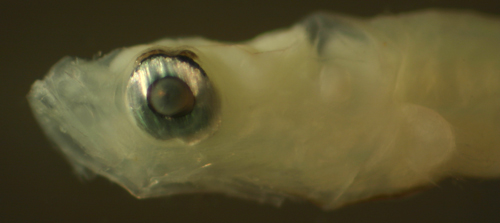
| Microgobius
signatus larva |
| 6.3 mm SL |
| note pigmented membrane
above eyeball |
| San Blas, Panama SB87-201 |
|
 |
| Microgobius
signatus larva |
| 8.5 mm SL |
| note pigmented membrane
above eyeball |
| San Blas, Panama SB87-228 |
|
 |
| Microgobius
signatus larva |
| 8.9 mm SL |
| note melanophore streak
onto pelvic fins |
| San Blas, Panama SB86-503 |
|
 |
| Microgobius
signatus larva |
| 9.4 mm SL |
| note pigmented membrane
above eyeball |
| San Blas, Panama SB86-1224 |
|
 |
| Microgobius
signatus larva |
| 10.0 mm SL |
| San Blas, Panama SB86-422 |
|
 |
| Microgobius
signatus larva |
| 11.9 mm SL |
| San Blas, Panama, SB86-502 |
|
 |
|
|
 |
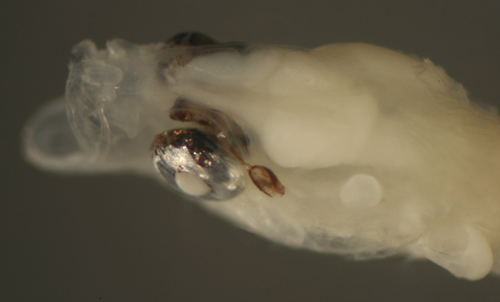
| Microgobius
signatus larva |
| 8.0 mm SL |
bilateral eye abnormality:
intracranial
and extraorbital extension of the eye |
| San Blas, Panama, SB84-624a |
|
 |
| |
 |
| Microgobius
sp.larva |
| 5.4 mm SL |
| earlier stage larva
eye abnormality |
| San Blas, Panama, SB84-526 |
|
 |
| |
 |
|
|
|
|
|
|
|
|
| Diagnosis:
An individual larva with fin-ray counts
of D-VII,21 A-21 Pect-18 indicates Palatogobius
paradoxus (Mauve Goby) and overlaps the range
for Microgobius
signatus. The second-dorsal-fin count for
P. paradoxus varies in the literature, but
the upper range is close at 20 elements (no other
goby has as high as 21 anal-fin elements). This
larval type furthermore shares the particularly
large round eye with P.
paradoxus (indicative of a deeper-water species),
as well as the fused pelvic fins, but without a
frenum. The bathydemersal sibling species P.
grandoculus cannot be excluded. Ptereleotris
helenae (previously Ioglossus
helenae) modally has 22 or more second-dorsal-fin
elements (and only six first-dorsal-fin spines and
separate pelvic fins). (U) |
|
| Analogues:
(long goby with long dorsal and anal fins)
This larval type has unique markings for the group,
especially a melanophore below the base of the mid-dorsal
fin (in a pre-transitional larva). Body form similar
to Microgobius,
but no anal-fin row of melanophores and a distinctly
longer pelvic fin. |
|
| Description:
Body thin, long, and narrow with a very large eye
and a large terminal mouth. Pectoral fins medium
length, pelvic fins long, reaching most of the way
to the anus without an obvious frenum. Dorsal and
anal-fin bases long, caudal peduncle short and narrow,
8-9 procurrent caudal-fin rays (8 spindly). Lightly
marked; a row of streak melanophores on the ventral
midline from the isthmus to the pelvic-fin insertion
and one just behind the pelvic-fin insertion. There
are only two other melanophores on the body: one
at the base of the anal fin around the thirteenth
fin element and one at the dorsal midline below
the base of the sixth fin element. There are no
markings on the fins. Internal melanophores are
present at the dorsal surface of the swim bladder
and around the gut near the anus. The eye is large
and round with a prominent speckled "eyebrow" membrane
over the upper half of the eyeball that appears
detached from the pigmented iris below. |
|
|
|
| Palatogobius
paradoxus larva |
| 9.5 mm SL |
| San Blas, Panama, SB81-019 |
| |
|
 |
| |
 |
| |
 |
| |
 |
|
|
|
|
|
|
|
|
|
| Diagnosis:
Modal fin-ray counts of D-VI,23 A-22 Pect-21
indicate Ptereleotris
helenae (Hovering Goby or Hovering Dartfish)
or P. calliurus
(Blue Dartfish). This group of dartfishes has been
moved around taxonomically recently and presently
reside in Microdesmidae
with the wormfishes, although they appear more similar
to gobioids in general appearance, and as larvae.
The fin-ray counts are unique to this genus in the
region, where no other gobioid exceeds 22 second-dorsal-fin
elements (some Microgobius
signatus individuals can occasionally reach
22 elements and larval Palatogobius
have up to 21). In addition, these latter candidates
have seven first-dorsal-fin spines and do not have
the obviously separate pelvic fins of Ptereleotris.
There are two regional species: P. helenae
throughout most of the Caribbean and the sibling
species P. calliurus from Florida and the
Gulf of Mexico. (U)g21 |
|
| Analogues:
(long "goby" with long dorsal and anal
fins) Larval Ptereleotris
helenae have unique markings when compared
to the gobioids, especially a long row of melanophores
along the base of the dorsal fins in a pre-transitional
larva. Their body form is similar to larval Microgobius,
but the melanophore patterns are quite different.
The markings of larval Ptereleotris
helenae are similar to those found in some
larval labrisomids
or chaenopsids,
but larvae of those families do not have the distinctive
separate and short spinous dorsal fins of gobioids
and Ptereleotris. |
|
| Description:
Body thin, long and narrow with a
large eye and a large terminal mouth. Pectoral
fins short, pelvic fins medium-length, extending
less than halfway to the vent, clearly separate
with no frenum. Dorsal and anal-fin bases
very long, caudal peduncle very short and
narrow, procurrent caudal-fin rays 7-9 (7-8
spindly). Lightly marked along the dorsal
and ventral midlines: melanophores in rows
on the body near the base of the spinous dorsal
fin, variably paired and offset from the midline,
then in rows near the base of the second dorsal
fin, one offset pair per fin element, then
extending onto the dorsal midline at the caudal
peduncle ending at the start of the procurrent
caudal-fin rays. Melanophores are present
on some of the central and lower segmented
caudal-fin rays. There is a row of melanophores
along the anal-fin base (variably paired,
one per side, a few larger ones starting at
the fifth or sixth element that are fewer
than one per ray, then becoming small and
usually one per ray around the tenth element),
and then a streak along the ventral midline
of the very short caudal peduncle ending at
the start of the procurrent caudal-fin rays.
There are no melanophores at the isthmus or
at the pelvic-fin insertion. On the head there
is a pair of large melanophores at the rear
of the braincase on each side of the dorsal
midline. Internal melanophores are present
around the sacculus, along the dorsal surface
of the swim bladder and around the gut near
the vent. The eye is large and round. |
|
|
|
| Ptereleotris
helenae larva |
| 12.2 mm SL |
| note separated
pelvic fins |
| San Blas, Panama,
SB82-016 |
|
 |
| |
 |
| |
 |
| |
 |
| |
 |
|
|
|
| |
|
 |
|
All contents copyright 2006-2018
All rights reserved
www.coralreeffish.com by
Benjamin Victor
|
|
|
|
| |