|
|
|
|
Group 3: The seven-spined shortfin
gobies (fused)
|
|
Barbulifer,
Risor,
Ginsburgellus,
Gobiosoma,
Elacatinus,
and Tigrigobius
|
| |
|
This group includes many of the small gobies
on and around Caribbean reefs that live
well-hidden around coral structure or inside
sponges. Most are inconspicuous and rarely
noticed on the reef. The main exception
is the group of cleaner gobies that live
on prominent coral heads and sponges and
remove parasites from passing fishes. They
need to advertise and typically have bright
blue or yellow stripes on a black background.
Interestingly, a set of related sponge gobies
share the colored stripe, but do not apparently
clean other fishes; the reason for their
colors could either be to receive some protection
from the relative immunity of cleaners from
predation (mimicry) or advertise the fact
that they produce noxious chemicals. The
striped sponge gobies usually stay in their
sponges and do not perch in conspicuous
locations as do the cleaners.
A group of small non-descript inshore,
sometimes even freshwater, gobies are also
in this group. The phylogenetic relationships
are not resolved and some species have been
shuttled around into various genera over
the years. The most recent change has been
the returning of the non-cleaner/sponge
gobies of Elacatinus
back to Tigrigobius,
where they form a cohesive grouping.
|
| |
| The
larvae of Group 3 gobies are typically very
small and lightly marked, usually with only
a few ventral midline melanophores or often
just a single post-anal-fin spot. The basic
shortfin meristics (usually 8-11 second-dorsal
and anal-fin elements) and general appearance
are shared by some larvae of the six-spined
standard gobies of Group
2 and the two groups can be a challenge
to separate when the dorsal-fin spines are
not easily apparent. Similarly, some of the
divided pelvic-fin gobies, Group
5, have larvae that are similar in size,
shape, and markings to the Group 3 gobies
and they also can be difficult to distinguish
when the state of the pelvic fins is not obvious.
The few Group 3 gobies with 13 second dorsal-fin
elements overlap the lower range of fin counts
of the longfin gobies of Group
4, but have a quite different body shape
and larval appearance. Only the occasional
Gobiosoma
from US waters have counts that high, but
they notably have no more than 11 anal-fin
elements. |
|
|
|
| |
|
|
|
|
|
| Diagnosis:
Fused pelvic fins and modal fin-ray counts
of D-VII,10 A-9 and Pect-16-17 indicate Barbulifer
species. Other seven-spined gobies with this modal
fin-ray count have divided pelvic fins and include
Pycnomma
roosevelti, Psilotris,
and Chriolepis.
Gobiosoma grosvenori
shares the fin-ray counts and should have a mostly-divided
pelvic fin but, since the rest of the Gobiosoma
have typical fused pelvic fins, the pelvic
fin in larval G. grosvenori
may not be divided (it also occurs only in Florida
and Venezuela). This larval type, however, has the
shape characteristic of Barbulifer,
i.e. a large broad and flattened head, a wide mouth,
a short pelvic fin with a frenum, and a wide caudal
peduncle. There are two Caribbean species with the
same fin-ray counts: B.
ceuthoecus and
B. antennatus. The latter is not reported
from Panama. The remaining seven-spined gobies all
have a mode of more than 10 second-dorsal-fin elements.
(PE) G18 |
| |
| Analogues:
|
| |
| Description:
Body thick, long, and somewhat narrow with
a small eye and a terminal large wide mouth. Head
broad and slightly flattened. Pectoral fins relatively
long, reaching much of the way to the vent. Pelvic
fins short, extending less than halfway to the vent
and fused with a small frenum (some larvae show
split pevic fins that are artifacts). Dorsal and
anal-fin bases short, caudal peduncle relatively
wide and long and procurrent caudal-fin rays 8-10
(8-9 spindly). Melanophores on the head are limited
to at the angle of the jaw and sometimes on the
inner (mouth) side of the lower jaw. Characteristically,
there is a large melanophore or two overlying the
cleithrum just behind the operculum and just forward
of the pectoral-fin base. This melanophore can be
absent on very lightly-marked individuals. Lightly
marked, mostly along the ventral midline: at the
isthmus, just forward of the pelvic-fin insertion,
then sometimes on the abdomen just behind the pelvic-fin
insertion, then three or four melanophores spaced
along the anal-fin base (often paired, one per side)
and then a row (or streak) of three or four extending
along the caudal peduncle ending near the start
of the procurrent caudal-fin rays. The abdominal
melanophores are quite variable, missing in some
individuals and a clear Y-shape diverging from the
pelvic-fin insertion in others (post-pelvic Y).
Melanophores are present on the base of most of
the lower caudal-fin segmented rays extending up
to halfway along the rays. There are internal melanophores
around the sacculus, on the dorsal surface of the
swim bladder, and around the gut near the vent.
Series of transitional larvae show that the eye
develops from a slightly narrowed vertical oval,
sometimes tilted forward, to larger and round. Transitional
larvae always have the melanophore overlying the
cleithrum just forward of the base of the pectoral
fin, and often more around the base of the pectoral
fin. Melanophores then develop as a scattering in
a vague stripe on the body underlying the pectoral
fin rearwards. In addition, there can be melanophores
on the inside of the mouth at the tip of the lower
jaw and a few tiny ones around the maxilla. |
|
|
|
| Barbulifer
ceuthoecus larva |
| 9.0 mm SL |
| San Blas, Panama, SB83-169 |
|
 |
| Barbulifer
ceuthoecus larva |
| 9.3 mm SL |
| slightly narrowed eye |
| San Blas, Panama, SB87-218 |
|
 |
| |
 |
| |
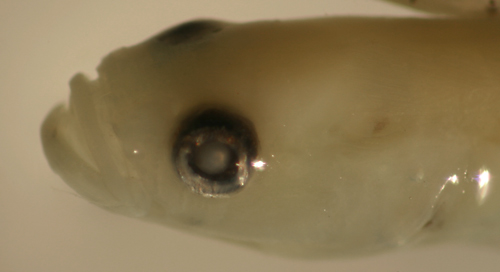
|
| Barbulifer
ceuthoecus larva |
| 9.9 mm SL |
| lightly marked |
| San Blas, Panama, SB80-105 |
|
 |
| Barbulifer
ceuthoecus larva |
| 9.5 mm SL |
| broad head and inner
mouth melanophores |
| San Blas, Panama, SB87-228 |
|
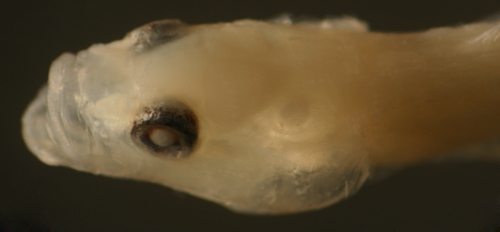 |
| |
 |
| Barbulifer
ceuthoecus transitional larva |
| 9.4 mm SL |
| San Blas, Panama, SB86-331 |
|
 |
| |
 |
| |
 |
| |
 |
|
|
|
|
|
Goby 1125 vs.
Barbulifer ceuthoecus (early) |
|
|
|
|
| Diagnosis:
A larval type with D-?,10 A-9. Unfortunately,
the 10/9 fin-ray count is the most common formula
for Caribbean gobies and there are many candidates
for this larval type. The basic melanophore pattern,
i.e. a melanophore at the angle of the jaw, a row
along the anal-fin base continuing to the start
of the lower procurrent caudal-fin rays and melanophores
at the base of most of the lower segmented caudal-fin
rays, is shared with larval Barbulifer
ceuthoecus and the six-spined Coryphopterus.
The body shape of this larval type, however, does
not match the Coryphopterus.
In most features, this larval type fits with what
would be expected for immature B.
ceuthoecus larvae, i.e. the pattern of melanophores
(especially the post-pelvic Y), the small round
eye, the flattened head shape with a very broad
mouth, and the relatively wide caudal peduncle.
However, the melanophore just forward of the vent
on the abdominal promontory is not found on other
B.
ceuthoecus larvae, and this larval type
is therefore described separately (pending intermediate
individuals or DNA sequencing). Barbulifer antennatus
is not reported from Panama, but cannot be excluded. |
|
| Analogues:
(light markings with anal fin plus caudal peduncle
row) Within the diverse anal fin plus caudal
peduncle row group, there are many very similar
larval types. Two of the most common gobiid larval
genera share this basic marking pattern, including
the melanophore at the angle of the jaw: Lythrypnus
(without the caudal-fin melanophores) and Coryphopterus
(both six-spined). A few Elacatinus
are the only seven-spined gobies to share the anal-fin-caudal
peduncle row of melanophores, but, as a rule, they
do not have the melanophore at the angle of the
jaw. All three of the aforementioned groups are
typically wider-bodied and do not share the flattened
head appearance and broad mouth of this larval type.
Furthermore, their eyes are either narrowed vertical
ovals or large and round, without the smaller slightly
flattened eye exhibited by this larval type. They
do not share the post-pelvic Y marking and only
a rare Coryphopterus
specimen exhibits an abdominal promontory melanophore.
Typical Barbulifer
ceuthoecus larvae are larger, usually more
than 9 mm SL, and thicker (but may represent the
mature version of this larval type). |
|
| The
larval eleotrid Dormitator
maculatus has a similar general appearance
and shares most of the markings, including the abdominal
promontory and jaw angle melanophores, but the abdominal
midline streak extends to the level of the swimbladder
(shared with the other eleotrid species) and there
is no internal melanophore around the gut near the
vent. Some immature larvae of the long gobies, such
as
Microgobius superficially resemble this
type, but have many more median-fin rays and very
short caudal peduncles and are usually longer than
8 mm SL. Immature Bollmannia
boqueronensis larvae may resemble this type,
but have more median-fin rays and a much larger
irregular eye, along with additional melanophores.
|
|
| Description:
Body relatively thick, long, and narrow with
a medium round eye and a terminal large wide mouth.
Head broad and slightly flattened. Dorsal and anal-fin
bases short, caudal peduncle relatively wide and
long and procurrent caudal-fin rays 8-9 (8 spindly).
Lightly marked, mostly along the ventral midline:
at the isthmus, along the pelvic-fin insertion and
extending onto the abdominal midline, often with
a clear Y-shape diverging from the pelvic-fin insertion
(post-pelvic Y), then often a melanophore on the
abdominal promontory just forward of the vent, followed
by paired melanophores along the anal-fin base and
then a row (or streak) extending along the caudal
peduncle ending near the start of the procurrent
caudal-fin rays. Melanophores are present on the
base of most of the lower caudal-fin segmented rays.
Melanophores on the head are limited to the angle
of the jaw. There are internal melanophores along
the dorsal surface of the swim bladder and around
the gut near the vent. |
|
|
|
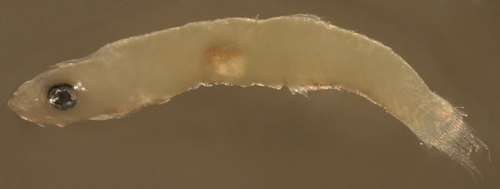
| Goby
1125 larva |
| 7.4 mm SL |
| San Blas, Panama, SB86-1125 |
|
 |
| |
 |
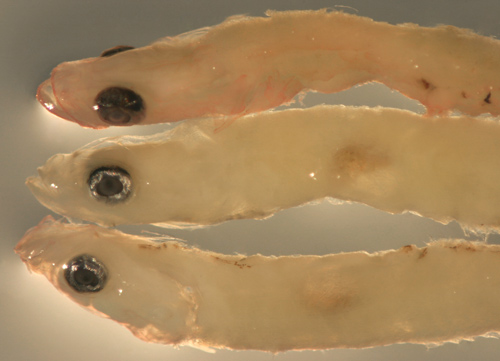
| Goby
1125 larvae |
| 7.1, 7.4, and 8.0 mm
SL |
larva at bottom shown
ventral aspect up
with a post-pelvic Y and an abdominal
promontory melanophore |
| San Blas, Panama, SB86-1125 |
|
 |
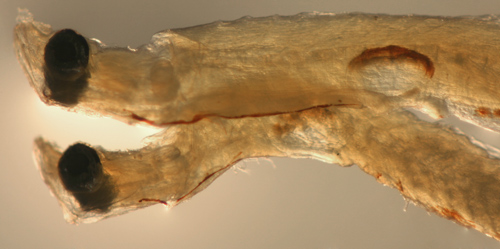
Dormitator
maculatus larva above
vs. Goby 1125 larva below |
note abdominal midline
melanophores,
long streak vs. Y |
| San Blas, Panama, SB86-1123 |
|
|
|
|
|
|
|
Notes on
Elacatinus, Tigrigobius, and Gobiosoma |
|
|
|
|
| This
large group of small seven-spined gobies separates
out into three basic groups: the conspicuously-striped
Elacatinus cleaner and sponge gobies (also
commonly called neon gobies), the non-cleaner Tigrigobius,
and Gobiosoma. Identification of larvae to
species in this large and homogenous group is obviously
difficult and DNA sequencing is necessary to be
sure of the identification for some individuals.
The larvae of these seven-spined goby larvae are
similar in general appearance to those of some six-spined
species, especially Lythrypnus
and Coryphopterus,
but with the characters presented here they should
be easily separated. |
|
| Larval
identifications can be problematic since the fin-ray
counts of many species overlap (although modal counts
can be helpful). The cleaner/sponge gobies separate
out with their high median-fin ray counts, usually
D-VII,12 A-11. Unfortunately, many of the Tigrigobius
share fin-ray counts with the Gobiosoma,
making this large group of gobies particularly hard
to identify. Pectoral-fin ray counts can vary between
species and is sometimes useful. The most helpful
feature, however, is that many Gobiosoma
have restricted ranges within the region, thus knowledge
of the location of a collection can sharply reduce
the number of possible candidates and be instrumental
in identifications. |
|
| Taxonomy:
These gobies have a complex taxonomic history and
have been shuffled around a number of genera: the
present Tigrigobius
have, until very recently, been placed in Elacatinus.
The present Gobiosoma group includes the
former Garmannia and Austrogobius.
The cleaner/sponge gobies, presently considered
Elacatinus, have been included in Gobiosoma
in the past and are often listed as such in
older literature. |
|
| Elacatinus
vs. Tigrigobius |
|
| Cleaner/sponge
group (neon gobies): The Elacatinus
cleaner/sponge gobies typically have higher modal fin-ray counts
than Tigrigobius,
i.e. D-VII,12 A-11. Geographic location is very
important to identification of these gobies. |
| |
| Tigrigobius
group: Although there is some variation,
modal fin-ray counts of D-VII,11 A-10 are typical
of the Tigrigobius. These species are typically
reef-associated, although often inconspicuous. Some
have broad geographic ranges, in contrast to most
cleaner/sponge gobies and the Gobiosoma species.
The fin ray counts are shared by many of the present
Gobiosoma (discussed separately below). |
|
|
|
The Tigrigobius
separate out somewhat by modal pectoral-fin
ray counts: T.
gemmatus, T.
saucrus and T.
pallens with 16, T.
dilepis and T. macrodon with
17 (the latter Florida to Haiti), T. zebrellus
with 18 (Venezuela and Trinidad only), and
T.
multifasciatus complex of species
with 20-21 pectoral-fin rays. |
|
|
|
Tigrigobius
exceptions to the standard D-VII,11 A-10
fin-ray count are T.
pallens with a modal fin-ray count
of D-11 A-9, many T.
multifasciatus with D-12 A-10, and
some populations of T.
gemmatus with D-12 A-11.
The 11-12/10 median-fin ray count is shared
by two other species that are closely-related
to the Tigrigobius:
Ginsburgellus novemlineatus (Pect-17
and a tiny pelvic-fin cup), and Risor
ruber (D-11-12, A-9-10, Pect-15-17).
Both fall within the Tigrigobius
clade in phylogenetic studies.
|
|
|
| Gobiosoma |
| |
| The
present genus Gobiosoma includes a number
of tiny non-descript species, several limited to
US waters (G. bosc, G. ginsburgi, G. longipala,
and G. robustum). Most Caribbean species
have a modal fin-ray count of D-VII,11 or 12 A-10.
They are found in shallow inshore non-reef environments,
including brackish and fresh-water, and fortunately
can have very restricted geographic ranges which
makes identifications much easier. At most Caribbean
locations, only one or two species are to be expected,
but, for some interesting biogeographic reason,
three species coexist around Colon in Panama. |
| |
|
|
Those Caribbean species
with a mode of 11/10 include G.
hildebrandi (from Panama) with modal
17 pectoral-fin rays and G. schultzi
(from Lake Maracaibo, Venezuela) with 17-18
pectoral-fin rays. Note: these fin-ray counts
can be shared with several Tigrigobius
species discussed above. |
|
|
|
Those with a mode of 12/10 include G.
spes (Pect-16, widespread), G.
spilotum (Pect-18-19, Panama), and G.
hemigymnum (Pect-20-21, West Indies).
|
|
|
|
|
Caribbean Gobiosoma
exceptions to the standard D-VII,11 or 12
A-10 fin-ray counts are G. grosvenori
(S. Florida, Bahamas, and Venezuela only)
with modally D-10 A-9 (shared among the seven-spined,
fused-pelvic-fin gobies by the two Barbulifer
species; all three with modal 17 pectoral-fin
rays) and G. yucatanum (Yucatan to
Honduras) with D-11 A-9 Pect-15-16 (shared
with E.
pallens).
|
|
|
|
|
The USA and Gulf of Mexico
Gobiosoma species can have higher
median-fin ray counts of D-VII,12 or 13 A-11
Pect-17-19 in G.
bosc and G. ginsburgi (shared
by many cleaner gobies) or the same as Caribbean
species (D-VII,11 or 12 A-10 Pect-15-17) in
G. longipala and G. robustum. |
|
|
| Elacatinus/Gobiosoma vs.
the six-spined gobies |
|
| Many
Elacatinus/Gobiosoma larvae superficially
resemble those of Lythrypnus
and Coryphopterus
(both six-spined). Since the latter are
very common larvae and the number of dorsal-fin
spines is not always easily apparent, the groups
can be easily confused. There are, however, several
basic features that should quickly serve to separate
the groups (other than counting dorsal-fin spines):
|
|
|
|
the row of melanophores
along the ventral midline of the caudal peduncle
extends close to, or up to, the start of the
procurrent caudal-fin rays in Coryphopterus
and, with uncommon exceptions, in Lythrypnus.
In Elacatinus/Gobiosoma larvae, the
row, if present, stops well before the start
of the procurrent caudal-fin rays. |
|
|
|
|
The melanophore at the
angle of the jaw is present in all Lythrypnus
larvae and many Coryphopterus
larvae (i.e. the vast majority of
C.
glaucofraenum and C.
dicrus, but not in C.
personatus/hyalinus), but is absent
in all Elacatinus/Gobiosoma larvae
identified thus far. |
|
|
|
|
Coryphopterus
larvae have more procurrent caudal-fin
rays, usually 8 or 9, than do Elacatinus/Gobiosoma
larvae (or Lythrypnus),
who usually have only 5 or 6. |
|
| |
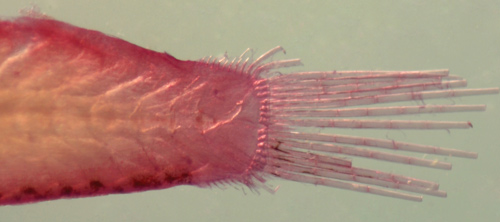
| Of course,
this discussion is limited to the larval taxa so
far identified. Photographs below of the tails of
larval C.
glaucofraenum above and Tigrigobius gemmatus below. |
| |
 |
|
 |
|
|
|
|
Note: three genera combined, presented
in order of increasing anal-fin elements
|
|
|
|
|
|
|
|
| Diagnosis:
Fused pelvic fins and modal fin-ray counts of D-VII,11
A-8-9 and Pect-16 indicate Tigrigobius pallens
and Gobiosoma yucatanum.
This low fin-ray count matches only a few species
in the large Gobiosoma/Tigrigobius/Elacatinus group.
The modal count overlaps the lower range for T. saucrus and T. dilepis. However, individuals of this larval
type range as low as D-VII,10 A-8 Pect-15. G.
yucatanum (Pect-16 with range 15-18) occurs
only from Yucatan to Honduras. Gobiosoma
grosvenori can overlap these lower counts,
but is found only in Florida, Bahamas, and Venezuela.
(PE) G7ab |
|
| Analogues:
(anal-fin base row and post-anal-fin spot only)
|
|
| Description:
Body relatively thin, long and narrow with
a large eye and a medium-sized low terminal mouth.
Pectoral fins long, reaching past the vent. Pelvic
fins long, reaching about two-thirds of the way
to the vent without an obvious frenum or cup. Dorsal
and anal-fin bases medium length, caudal peduncle
long and narrow and only 4-6 procurrent caudal-fin
rays (4-5 spindly). Very lightly marked along the
ventral midline: melanophores absent from thoracic
and pelvic region and present as a short row of
two to four along anal-fin base between the third
and seventh element (variably paired and variably
one per side) and then one large melanophore just
after the last anal-fin ray. Some individuals have
a surface melanophore just forward of the anal-fin
origin. Internal melanophores at the dorsal surface
of the swim bladder. Series of transitional larvae
show the eye remains round. Transitional larvae
first develop two arcs of melanophores across the
top of the head between the eyes, subsequently two
more arcs develop behind the eyes and then two bars
of melanophores develop below the eye (corresponding
iris melanophores at about 12, 2, 5, and 7 o'clock).
A scattering of tiny melanophores develops along
the upper jaw and around the nasal bones. |
|
|
|
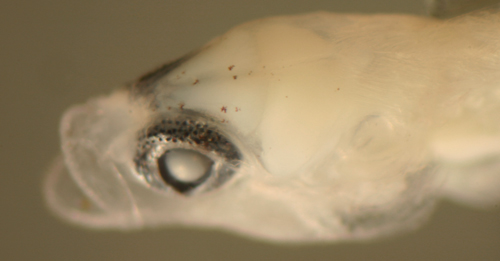
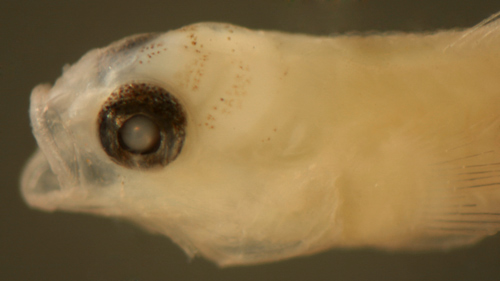
| Tigrigobius
pallens larva |
| 6.1 mm SL |
| early transitional
body shape? |
| San Blas, Panama, SB84-624a |
|
 |
| |
 |
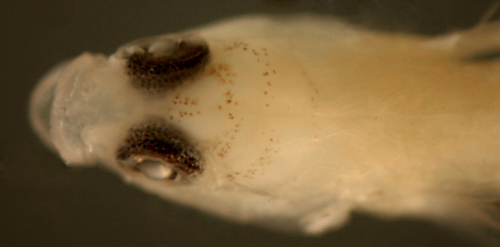
| Tigrigobius
pallens early transitional larva |
| 6.7 mm SL |
| San Blas, Panama, SB86-426 |
|
 |
| |
 |
| |
 |
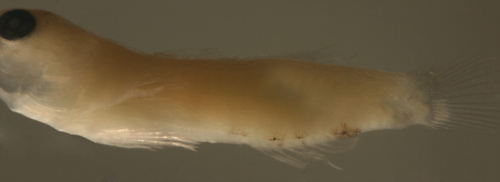
| Tigrigobius
pallens transitional larva |
| 7.0 mm SL |
| D-VII,10 A-8 Pect-16 |
| San Blas, Panama, SB86-228 |
|
 |
| |
 |
| |
 |
| |
 |
| |
 |
| Tigrigobius
pallens transitional larva |
| 6.0 mm SL |
| D-VII,10 A-8 Pect-15 |
| San Blas, Panama, SB86-625 |
|
 |
| |
 |
| |
 |
| Tigrigobius
pallens recruit |
| 8.0 mm SL |
| Utila, Honduras U870 |
|
 |
|
|
 |
|
|
|
|
|
|
|
|
| Diagnosis:
Fused pelvic fins and modal fin-ray counts of D-VII,11
A-10 and Pect-16 overlaps many species of the numerous
Gobiosoma/Tigrigobius/Elacatinus group, but matches the
mode only for Tigrigobius
saucrus, T. macrodon (Florida to Haiti),
and Gobiosoma
hildebrandi (Panama). Tigrigobius
dilepis shares most features with T.
saucrus, but has a mode of 17 or 18 pectoral-fin
rays; individuals may be included in the larval
type where the species overlap in geographical distribution
(but this larval type in Panama has 15-17 pectoral-fin
rays with a strong mode at 16). Transitional recruits
with a prominent row of black spots along the side
of the body confirms T. saucrus. G7 |
|
|
Analogues: (ventral
midline series x3: thorax, anal-fin, caudal peduncle
streak) Larval T. saucrus share this
melanophore pattern with several congeners:
T. dilepis larvae are likely identical,
but with one or two more pectoral-fin rays (modal
17-18); T.
multifasciatus have a much shorter cup-shaped
pelvic fin, less than half-way to the vent, and
20-21 pectoral-fin rays; and the cleaner
gobies have a larger eye and D-12, A-11 fin-ray
counts. Larval T. saucrus and congeners
can be separated from the very common six-spined
gobies with the same VMSx3 melanophore pattern
primarily by the length of the caudal peduncle
streak. Most larval Coryphopterus
and Lythrypnus
(all six-spined) have their caudal peduncle
streak extending to the start of the procurrent
caudal-fin rays vs. about half-way for the seven-spined
gobies. Many of those six-spined species also
have a prominent melanophore at the corner of
the jaw, absent on the seven-spined larvae. Also
distinctive is the large cup-shaped pelvic fin
on larval T. saucrus; the seven-spined
gobies tend to have flat pelvic fins.
|
|
| Description:
Body relatively thin, long, and narrow with
a medium eye and a terminal, medium-sized mouth
and often thick lips. Pectoral fins long, reaching
to vent, pelvic fins long and form a large obvious
cup extending about two-thirds of the way to the
vent. Dorsal and anal fins relatively short, caudal
peduncle long and narrow, procurrent caudal-fin
rays 6-8 (6-7 spindly). Lightly marked along the
lower body: melanophores along the ventral midline
at the isthmus (sometimes missing) and the pelvic-fin
insertion, along the anal-fin base (paired, one
per side) and extending along the ventral peduncle
ending well before the start of the procurrent caudal-fin
rays. Internal melanophores at the dorsal surface
of the swim bladder and sometimes around the gut
near the vent. Melanophores sometimes present on
the base of several of the lower caudal-fin segmented
rays and extending out a short distance along the
rays. Series of transitional larvae show the eye
remains round. Transitional larvae develop a blunt
snout profile and develop bars radiating from the
eye and bands across the top of the head, connected
by a large X pattern over the brain. Patches of
melanophores develop on the preopercle, on the base
of the pectoral fin, and in a prominent row along
the dorsal midline of the body as well as along
the lateral midline and around the base of the caudal-fin
rays. |
|
|
|
| Tigrigobius
saucrus larva |
| 7.8 mm SL |
| San Blas, Panama, SB83-161 |
|
 |
| |
 |
| Tigrigobius
saucrus + larva |
| 7.7 mm SL |
| 17 pectoral-fin rays |
| San Blas, Panama, SB86-414 |
|
 |
| |
 |
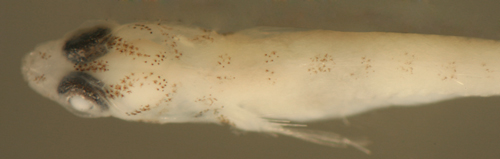
| Tigrigobius
saucrus + transitional larva |
| 7.6 mm SL |
| Pect-17 |
| San Blas, Panama, SB86-502 |
|
 |
| |
 |
| Tigrigobius
saucrus transitional larva |
| 7.9 mm SL |
| Pect-15 |
| San Blas, Panama, SB86-809 |
|
 |
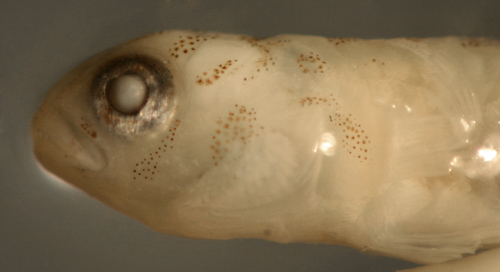
| Tigrigobius
saucrus transitional larva |
| 7.9 mm SL |
| Pect-16 |
| San Blas, Panama, SB87-121 |
|
 |
| Tigrigobius
saucrus late transitional larva |
| 7.7 mm SL |
| San Blas, Panama, SB86-1224 |
|
 |
| |
 |
| |
 |
|
|
|
|
|
|
|
|
| Diagnosis:
Fused pelvic fins and modal fin-ray counts of D-VII,11
A-10 and Pect-17 overlaps many species of the numerous
Gobiosoma/Elacatinus group, but matches the
mode only for the allopatric set of Gobiosoma
hildebrandi (Panama), G.
schultzi (Venezuela), G.
yucatanum (Yucatan to Honduras), and then
the pair of Tigrigobius saucrus and T. dilepis. G710 G7S1110 |
|
| Analogues:
(solitary post-anal-fin spot) Pre-transitional
Gobiosoma hildebrandi larvae can only be
distinguished from their co-ocurring congeners by
fin-ray counts (but there is overlap); the combination
of 11/10 p-17 vs. 12/10 p-16 in G.
spes and 12/11 p-16 in the T. gemmatus type. This group is distinguished
by having flat pelvic frenums vs. obvious cup-shaped
pelvic fins in T. saucrus and T. dilepis. At transition, G. hildebrandi
develop outlined scales along the posterior body
(vs. naked in newly-settled G.
spes). |
|
| Description:
Body relatively thin, long, and narrow with
a medium eye and a terminal, medium mouth. Pectoral
fins long, reaching to or past vent. Pelvic fins
medium, extending about two-thirds the way to the
vent, with a flat frenum not forming an obvious
cup. Dorsal and anal-fin bases medium length and
caudal peduncle medium length and width, procurrent
caudal-fin rays usually 6 (5-6 spindly). A single
melanophore on the caudal peduncle ventral midline
just after the last anal fin and internal melanophores
only at the dorsal surface of the swim bladder and
sometimes around the gut near the vent. |
|
|
|
| Gobiosoma/Elacatinus
sp. larva |
| 5.8 mm SL |
| D-VII,11 A-10 |
| San Blas, Panama, SB86-419 |
|
 |
| |
 |
| |
 |
| |
 |
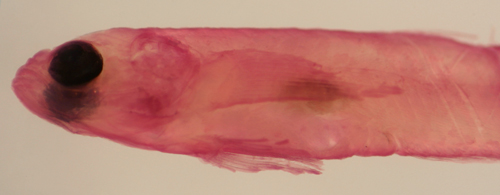
| Gobiosoma
hildebrandi recruit |
| 8.4 mm SL |
| Colon, Panama, N762a |
|
 |
|
|
|
|
|
|
|
|
| Diagnosis:
Fused pelvic fins and modal fin-ray counts of D-VII,12
A-10 and Pect-15-17 overlaps many species of the
numerous Gobiosoma/Elacatinus group, but
matches the mode only for Gobiosoma spes,
Tigrigobius gemmatus, Ginsburgellus
novemlineatus, and Risor
ruber. The pre-transitional larvae of these
species may be similar, but can be distinguished
at transition: T. gemmatus
should have basicaudal scales (G. spes do
not, only the sides of the body have some scales),
Ginsburgellus
novemlineatus should have a very small pelvic-fin
cup, and Risor
ruber has a long pelvic fin and a row of
distinctive spiny scales on the ventral midline
of the caudal peduncle. G419 G7S1210 |
|
| Analogues:
(solitary post-anal-fin spot) Pre-transitional
Gobiosoma spes larvae can only be distinguished
from their co-ocurring congeners by fin-ray counts;
the combination of 12/10 p-16 vs. 11/10 p-17 in
G.
hildebrandi and 12/11 p-16 in the T. gemmatus type. This group is distinguished
by having flat pelvic frenums vs. obvious cup-shaped
pelvic fins in T. saucrus and T. dilepis. At transition, this type does not
develop ctenoid basicaudal scales as in T. gemmatus (and maybe G.
hildebrandi). |
|
| Description:
Body relatively
thin, long, and narrow with a medium eye and a terminal
medium mouth. Pectoral fins long, reaching to or
past vent. Pelvic fins medium, extending about two-thirds
the way to the vent, with a flat frenum not forming
an obvious cup. Dorsal and anal-fin bases medium
length and caudal peduncle medium length and width,
procurrent caudal-fin rays usually 6 (5-6 spindly).
A single melanophore on the caudal peduncle ventral
midline just after the last anal fin and internal
melanophores only at the dorsal surface of the swim
bladder and sometimes around the gut near the vent. |
|
|
|
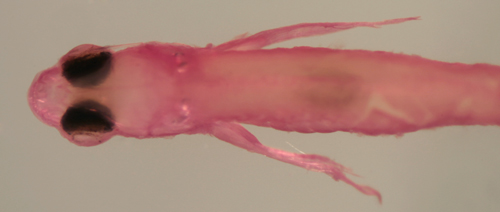
| Gobiosoma/Elacatinus
sp. larva |
| 6.6 mm SL |
| D-VII,12 A-10 Pect-17 |
| San Blas, Panama, SB86-808 |
|
 |
| Gobiosoma
spes transitional recruits |
| 6.6 mm SL top, 6.1
mm SL |
| D-VII,12 A-10 Pect-16,
DNA confirmed ID |
| Colon, Panama, N762a |
|
 |
| Gobiosoma
spes transitional recruits |
| 6.6 mm SL top, 6.1
mm SL, 5.9 mm SL |
| D-VII,12 A-10 Pect-16,
DNA confirmed ID |
| Colon, Panama, N762a |
|
 |
| Gobiosoma
spes transitional recruits |
| 6.6 mm SL |
| Note flattened pelvic-fin
frenum |
| Colon, Panama, N762a |
|
 |
| |
 |
|
|
|
|
|
|
|
|
|
Diagnosis: Fused
pelvic fins and modal fin-ray counts of D-VII,12
A-11 and Pect-16 and fused pelvic fins match the
Elacatinus
cleaner
gobies as well as overlapping the reported
upper range of T. gemmatus (and T. macrodon
from Florida to Haiti) and Gobiosoma
spes. In US waters, several Gobiosoma
species also fit this fin-ray count. Many
larvae of this type in Panama have a pair of spiny
basicaudal scales (ruling out the cleaner
gobies and G.
spes), leaving only T. gemmatus.
In Panama, 2 out of 3 larvae of this type have
12/11 p-16 (remainder mostly 12/10). G7S1211
|
|
| Analogues:
(solitary post-anal-fin spot) Pre-transitional
larvae before they develop basicaudal scales can
only be distinguished by modal fin-ray counts (12/11
p-16) from many other species: Gobiosoma
spes has mostly 12/10, Risor
ruber and
G. hildebrandi (Panama) have mostly 11/10.
The eyes on this larval type are smaller than those
of many related species. Many individuals have basicaudal
scales; these are absent from other larvae (uncertain
for
G. hildebrandi (Panama)). Risor
ruber larvae do not have the basicaudal
scales at transition, but do have distinctive spiny
scales along the ventral midline of the caudal peduncle.
Evermannichthys
larvae also have ventral midline scales along the
caudal peduncle, along with a very pointed snout
and and low pectoral-fin ray counts. Psilotris
batrachodes larvae have divided pelvic fins
and only 7-8 anal-fin elements. Psilotris
alepis larvae have divided pelvic fins,
a wider caudal peduncle, and a pelvic-fin base spot. |
|
| Description:
Body relatively thin, long, and narrow with
a medium eye and a terminal medium mouth. Pectoral
fins long, reaching to or past vent. Pelvic fins
medium, extending about two-thirds the way to the
vent, with a flat frenum not forming an obvious
cup. Dorsal and anal-fin bases medium length and
caudal peduncle medium length and width, procurrent
caudal-fin rays usually 6 (5-6 spindly). A single
melanophore on the caudal peduncle ventral midline
just after the last anal fin and internal melanophores
only at the dorsal surface of the swim bladder and
sometimes around the gut near the vent. The eye
is slightly vertically-narrowed and has a coarsely-speckled
membrane overlying the upper iris. Many larvae have
a pair of large spiny scales on the tail over the
base of the uppermost and lowermost segmented caudal-fin
rays. Transitional larvae first develop a sparse
scattering of small discrete melanophores on the
upper head, then a pair of melanophores behind the
tip of the upper jaw, a few on the anterior upper
jaw and an incipient stripe from the eye to the
mid upper-jaw. |
| |
|
|
|
| Tigrigobius gemmatus
larva |
| 6.3 mm SL |
| San Blas, Panama, SB86-502 |
|
 |
| |
 |
| Tigrigobius gemmatus
larva |
| 6.5 mm SL |
| no surface melanophores |
| San Blas, Panama, SB86-425 |
|
 |
| |
 |
| Tigrigobius gemmatus
larva |
| 7.2 mm SL |
| D-VII,12 A-11 Pect-18 |
| San Blas, Panama, SB86-502 |
|
 |
| Tigrigobius gemmatus
transitional larva |
| 6.4 mm SL |
| D-VII,12 A-11 Pect-15 |
| San Blas, Panama, SB87-228 |
|
 |
| |
 |
| |
 |
| |
 |
| |
 |
| Tigrigobius gemmatus
transitional larva |
| 6.2 mm SL |
| D-VII,12 A-11 Pect-16 |
| San Blas, Panama, SB86-709 |
|
 |
| Tigrigobius gemmatus
transitional larva |
| 6.5 mm SL |
D-VII,11 A-10 Pect-17,
basicaudal scales
head melanophores, bar from eye to jaw |
| San Blas, Panama, SB86-710 |
|
 |
| |
 |
| |
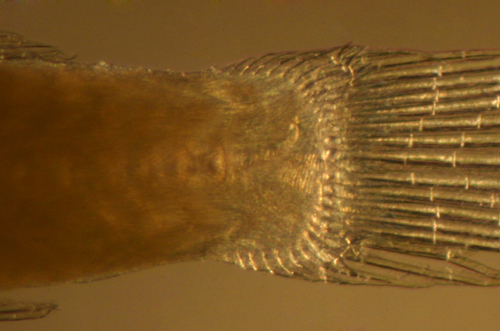 |
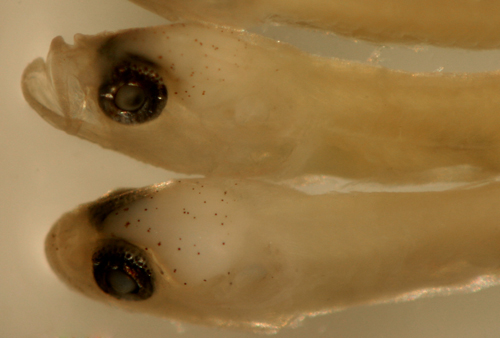
| Tigrigobius gemmatus
transitional larvae |
| 6.2 mm SL top, D-VII,12
A-11 Pect-16 |
| 6.5 mm SL lower, D-VII,11
A-10 Pect-17 |
| San Blas, Panama, SB86-710 |
|
 |
|
|
|
|
|
|
|
|
|
Diagnosis: Fused
pelvic fins and modal fin-ray counts of D-VII,11-12
A-10 and Pect-15-16 overlaps many species of the
numerous Gobiosoma/Elacatinus group and
matches quite a few: Risor ruber,
Ginsburgellus novemlineatus, Tigrigobius gemmatus and T. saucrus, as well as Gobiosoma
spes, G.
hildebrandi (Panama), G.
schultzi (Venezuela), and G.
yucatanum (Yucatan to Honduras). Fin ray
counts are variable in Risor ruber; my
Honduran specimens have D-VII,11 A-10 Pect-14-15.
The pre-transitional larvae of all of these gobies
are likely similar, but some species can be distinguished
at transition: both G.
hildebrandi and T. gemmatus should have distinct spiny basicaudal
scales; Ginsburgellus
novemlineatus have no scales and a very
small pelvic-fin cup; and Risor ruber have
a row of distinctive spiny scales on the ventral
midline of the caudal peduncle and long pelvic
fins. The enlarged scales are an apparent adaptation
to sponge-dwelling and also can be seen on the
transitional larvae of the other sponge gobies,
Evermannichthys.
The latter genus can overlap the median-fin ray
count for R. ruber, however they have notably
fewer pectoral-fin rays, usually only 12 or 13.
At transition, R. ruber becomes very distinct
morphologically, with a sharply-blunted snout
and uniquely curved and protruding fangs. (U)
|
|
| Analogues:
(anterior anal-fin base and caudal peduncle spot
only) This larval type has only one melanophore
at the anterior portion of the anal-fin base (thus
far the only specimen), while larval Elacatinus
pallens have two or more. Larval Evermannichthys
can also have the enlarged spiny scales on the ventral
caudal peduncle, but the spines are pigmented, there
are no anal-fin base melanophores, and the head
is pointed and the body more elongate. |
|
| Description:
Body thin, long, and narrow with a large
eye and a terminal small mouth. Pectoral fins long,
reaching to or past vent. Pelvic fins long, extending
almost to the vent, with a flat frenum not forming
an obvious cup. dorsal-fin base long, anal-fin base
medium and caudal peduncle medium, 5-6 procurrent
caudal-fin rays (4-5 spindly). There is a single
melanophore on the body below the base of the third
and fourth anal-fin elements and one or two melanophores
on the ventral midline of the caudal peduncle just
after the last anal-fin ray. Internal melanophores
occur only along the dorsal surface of the swim
bladder. There are three to four prominent spiny
scales along the ventral midline of the caudal peduncle
extending up to the start of the lower procurrent
caudal-fin rays. At settlement, transitional R.
ruber develop a markedky blunted snout with
a subterminal mouth containing a set of peculiar
outwardly-curved teeth. |
|
|
|
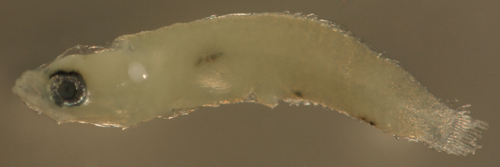
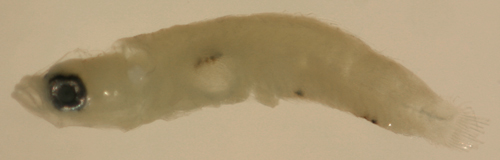
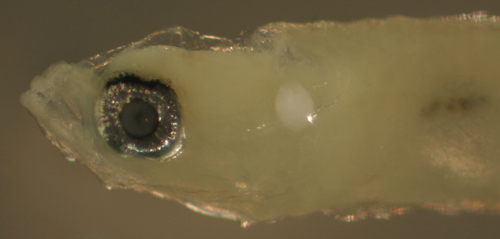
| Risor
ruber larva |
| 4.7 mm SL |
note ventral caudal
peduncle spiny scales
D-VII,12 A-10 Pect-16 |
| San Blas, Panama, SB87-201 |
|
 |
| |
 |
| |
 |
| |
 |
| Risor
ruber transitional recruit |
| 6.9 mm SL |
| spiny scales along
the caudal peduncle |
| Utila, Honduras U870 |
|
 |
| |
|
| |

|
|
|
|
|
|
| Ginsburgellus novemlineatus |
|
|
|
|
| Diagnosis:
Fused pelvic fins and modal fin-ray counts of D-VII,11-12
A-10 and Pect-15-17 overlaps many species of the
numerous Gobiosoma/Elacatinus group and matches
quite a few: Ginsburgellus novemlineatus,
Risor
ruber , Tigrigobius gemmatus and Tigrigobius saucrus, as well as Gobiosoma
spes, G.
hildebrandi (Panama), G.
schultzi (Venezuela), and G.
yucatanum (Yucatan to Honduras). The pre-transitional
larvae of all of these gobies are likely similar,
but some species can be distinguished at transition:
both G.
hildebrandi and T. gemmatus should have distinct spiny basicaudal
scales; Risor
ruber have a row of distinctive spiny scales
on the ventral midline of the caudal peduncle and
long pelvic fins. Larval Ginsburgellus novemlineatus
should have no basicaudal scales and may have a
small pelvic fin cup. |
|
| Analogues:
(solitary post-anal-fin spot) |
|
| Description:
|
|
|
|
| Ginsburgellus
novemlineatus? larva |
| 6.8 mm SL |
| D-VII,12 A-10 Pect-16,
esp. short pelvics? |
| San Blas, Panama, SB86-810 |
|
 |
|
|
|
|
|
| Tigrigobius multifasciatus,
panamensis, rubrigenis |
|
|
|
|
| Diagnosis:
Fused pelvic fins and modal fin-ray counts of D-VII,11-12
A-10 and the high pectoral-fin ray count of 20-21
indicates the Tigrigobius
multifasciatus species complex, comprising
T. multifasciatus in the Bahamas and Antilles,
T. panamensis in Panama and T. rubrigenis
in the Bay of Honduras. Gobiosoma hemigymnum
shares the high pectoral-fin ray count, but has
12-13 second-dorsal-fin elements. Gobiosoma
spilotum (Panama Canal) can also overlap,
but has a mode of 19 pectoral-fin rays. Gobiosoma
nudum, a Pacific species reported in the Caribbean
only near the mouth of the Panama Canal, has 20
pectoral-fin rays (18-20), but should have 12-13
second-dorsal-fin elements, not often 11 as in this
larval type. (PE) G405 |
|
| Analogues:
(ventral midline series x3: thorax, anal fin,
caudal peduncle streak) Larval T. multifasciatus
share this melanophore pattern with several congeners,
but have many more pectoral-fin rays and a short
cup-shaped pelvic fin extending less than half-way
to the vent. The congeners,
T. saucrus,
T. dilepis, and the cleaner
gobies have distinctly longer and prominent
cup-shaped pelvic fins. Larval E. multifasciatus
and the congeners can be separated from the very
common six-spined gobies with the same VMSx3 melanophore
pattern primarily by the length of the caudal peduncle
streak. Most larval Coryphopterus
and Lythrypnus
(all six-spined) have their caudal peduncle
streak extending to the start of the procurrent
caudal-fin rays vs. about half-way for the seven-spined
gobies. Many of those six-spined species also have
a prominent melanophore at the corner of the jaw,
absent on the seven-spined larvae. Also distinctive
is the cup-shaped pelvic fin on larval E. multifasciatus;
the seven-spined gobies tend to have flat pelvic
fins. |
|
| Description:
Body thin, long and somewhat narrow with
a large eye and a terminal mouth. Pectoral fins
fins long, pelvic fins form an obvious short cup
(a protruding frenum) extending less than halfway
to the vent. Dorsal and anal-fin bases relatively
short, caudal peduncle relatively long and narrow.
Lightly marked along the lower body: melanophores
along the ventral midline at the pelvic-fin insertion
(rarely also at the isthmus), along the anal-fin
base (paired, one per side) and extending along
the ventral peduncle ending well before the start
of the procurrent caudal-fin rays. Internal melanophores
at the dorsal surface of the swim bladder. |
|
|
|
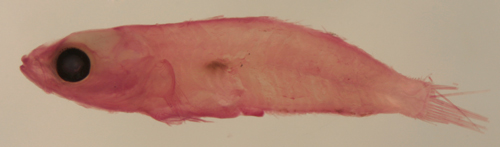
| Tigrigobius
panamensis larva |
| 6.9 mm SL |
| Pect-20 |
| San Blas, Panama, SB86-625 |
|
 |
| |
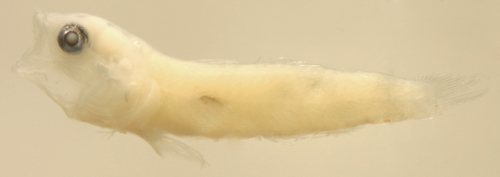 |
| Tigrigobius
panamensis larva |
| 7.6 mm SL |
| Pect-21 |
| San Blas, Panama, SB86-516 |
|
 |
| Tigrigobius
panamensis larva |
| 6.0 mm SL |
| Pect-21 |
| San Blas, Panama, SB86-405 |
|
 |
| |
 |
| |
 |
| |
 |
|
|
|
|
|
Notes on
the Elacatinus cleaner/sponge gobies (neon
gobies) |
|
|
|
| The
cleaner and the related striped sponge goby species
have a high modal median-fin ray count of D-VII,12
A-11, shared with few other related species. The
cleaner gobies are presently considered to be Elacatinus,
but have been considered Gobiosoma in the
past and are often listed as such in the literature.
Amongst the cleaners, there are few exceptions to
this modal median-fin ray count: E.
oceanops can have D-VII,13 and/or A-12,
E.
phthirophagus has D-VII,11 and E.
chancei has A-10. Pectoral-fin ray counts
vary but mostly overlap. Some Tigrigobius
gemmatus and the occasional specimens of
several other Elacatinus/Gobiosoma
would share the combination of 12 second-dorsal-fin
elements and 11 anal-fin elements. |
|
| It is
likely that larval cleaner/sponge gobies have a
similar or identical appearance and identification
would depend on location and DNA sequencing. Unlike
most other Caribbean reef fishes, the cleaner/sponge
gobies have restricted ranges to varying degrees
and thus location is critical for species identifications.
In addition, habitat is an important distinction,
with one set of species skating around on live coral
heads and usually abundant in shallow water and
another set living in and around sponges and often
in deeper water. The coral-associated cleaners are
typically more common and conspicuous on reefs and
include E.
evelynae, E. genie, E.
illecebrosus (not E. illecebrosum),
E.
oceanops, E. prochilos, E.
lobeli, and E. randalli. The deeper sponge
cleaner gobies are typically less conspicuous and
include E. chancei, E. horsti, E. xanthiprora,
E. colini, E. lori, E. louisae, and
E. tenox. Two very curious micro-endemic
species hover in groups over corals, quite unlike
the rest of the group: E. atronasus from
the Exuma Sound area of Bahamas and E. jarocho
from the Veracruz area of the Gulf of Mexico. |
|
| Adult
cleaner gobies are separated mostly by color patterns
and the shape of the snout. Some have a distinctly
underslung upper jaw, the true "sharknose"
appearance (E.
evelynae, E. genie, E.
illecebrosus, and E.
oceanops), but this character can be indistinct
on larvae and recruits. Color patterns on adults,
yellow vs. white vs. blue, are important characters
but also may be inconsistent on recruits. In general,
the most useful patterns for distinguishing new
recruits are the various markings on the snout. |
|
|
|
|
|
|
|
|
| Diagnosis:
Fused pelvic fins and modal fin-ray counts of D-VII,12
A-11 and Pect-16-17 indicate some of the cleaner
gobies of the genus Elacatinus
(formerly considered Gobiosoma). The common
cleaner goby in Panama is E.
illecebrosus, a bar-nosed species from the
south-western Caribbean. |
|
|
Analogues: (ventral
midline series x3: thorax, anal fin, caudal peduncle
streak) Larval cleaner gobies share this melanophore
pattern with several congeners: T. saucrus and
T. dilepis larvae have D-11 A-10 and a
smaller eye; T. multifasciatus have D-11 A-10, a shorter
cup-shaped pelvic fin, usually less than half-way
to the vent, and 20-21 pectoral-fin rays. Larval
cleaner gobies and congeners can be separated
from the very common six-spined gobies with the
same VMSx3 melanophore pattern primarily by the
length of the caudal peduncle streak. Most larval
Coryphopterus
and Lythrypnus
(all six-spined) have their caudal peduncle
streak extending to the start of the procurrent
caudal-fin rays vs. about half-way for the seven-spined
gobies. Many of those six-spined species also
have a prominent melanophore at the corner of
the jaw, absent on the seven-spined larvae. Also
distinctive is the large cup-shaped pelvic fin
on larval cleaner gobies; the six-spined gobies
tend to have flat pelvic fins.
Larvae of the cleaner gobies are likely identical,
but recruits and juveniles of E.
illecebrosus are separated from the other
bar-nosed species by having a wide-oval or wedge-shaped
pale area on top of the snout (not uniformly linear
as in E. randalli, E. colini,
E. xanthiprora, and E. lori) and a
mostly dark area from the eye forward to the upper
jaw (vs. pale in E. randalli and E.
colini).
|
|
|
Description: Body
thin, long, and narrow with a large eye and a
terminal, medium-sized mouth. Pectoral fins long,
reaching to vent, pelvic fins long and form a
large obvious cup extending about two-thirds of
the way to the vent. Dorsal and anal fins relatively
short, caudal peduncle long and narrow, tapering
rapidly and at the narrowest point, typically
only about the eye-diameter in width, procurrent
caudal-fin rays 6-8 (6-7 spindly). Lightly marked
along the lower body: melanophores along the ventral
midline at the isthmus (sometimes missing) and
the pelvic-fin insertion, then along the anal-fin
base (variable number, often only two or three)
and as a streak along the ventral caudal peduncle,
stopping well before the procurrent caudal-fin
rays. Internal melanophores at the dorsal surface
of the swim bladder and sometimes around the gut
near the vent.
Transitional recruits develop two wide dark stripes
along the top of the head separated by a thin
clear line. The stripes meet behind the head and
extend along the base of the dorsal fin fading
out before the caudal peduncle. The head stripes
extend forward onto the snout encircling a median
white bar that is distinctly oval, diamond- or
wedge-shaped. The dark area surrounding the bar
is uniform, not simply outlining the bar, and
covers most of the area between the eye and the
upper jaw, extending broadly onto the upper lip.
Below the dorsal midline stripe is a prominent
lateral clear band outlining a white iridescent
stripe, blue (or yellow) in life, running from
the dorsal aspect of the eyeball back to the upper
tail. Below the light band there is a wide dark
stripe running from the eye along the body just
below the lateral midline to the caudal fin, widening
at the caudal-fin base and then extending out
along the lower central caudal-fin rays. Deep
to this surface stripe is a broad streak of internal
melanophores extending above and below the lateral
midline.
|
|
|
|
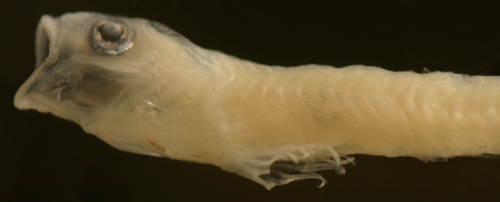
| Elacatinus
illecebrosus? |
| 8.3 mm SL |
| D-VII,12 A-11 Pect-17
|
| San Blas, Panama, SB86-808 |
|
 |
| |
|
| Elacatinus
illecebrosus transitional recruit |
| 8.7 mm SL |
| Colon, Panama, N7530a |
|
 |
| |
 |
| |
 |
| Elacatinus
illecebrosus transitional recruit |
| 8.7 mm SL |
| San Blas, Panama, SB81-021 |
|
 |
| Elacatinus
illecebrosus juvenile |
| 17.5 mm SL |
| sharknose appearance |
| San Blas, Panama, SB80-090 |
|
 |
|
|
|
|
|
|
|
|
| Diagnosis:
Fused pelvic fins and modal fin-ray counts of D-VII,12
A-11 and Pect-16-17 indicate some of the cleaner
gobies of the genus Elacatinus
(formerly considered Gobiosoma). The common
shallow-water cleaner goby in the Bay of Honduras
is E. lobeli, the recently-described neon
goby of the MAB, closely-related to the Florida
neon goby E. oceanops. This species, without
a bar or spot on the snout, is common along the
Meso-American Barrier Reef from Xcalak in Yucatan
across Belize to the Bay Islands of Honduras. |
|
| Analogues:
Larvae of the cleaner gobies are likely identical
(analogues discussed above under E.
illecebrosus), but recruits and juveniles
of E. lobeli are separated from the species
with V, bar, and spot markings on the nose tip by
having a uniformly dark dorsal snout area. |
|
|
Description: Larvae
of the cleaner gobies are likely identical (described
above under E.
illecebrosus). Transitional recruits develop
two wide dark stripes along the top of the head
separated by a thin clear line. The stripes meet
behind the head and extend along the base of the
dorsal fin fading out before the caudal peduncle.
Anterior to the head stripes, the dorsal snout
area is uniformly dark, covering most of the area
between the eye and the upper jaw. Below the dorsal
midline stripe is a prominent lateral clear band
outlining a white iridescent stripe (blue in life),
running from the dorsal aspect of the eyeball
back to the upper half of the tail. The snout
has a short extension of the blue stripe forward
of the eyes, but there is no meeting of the color
across the snout or midline bars or spots of color.
Below the light band there is a wide dark stripe
running from the eye along the body just below
the lateral midline to the caudal fin, widening
at the caudal-fin base and then extending out
along the lower central caudal-fin rays. Deep
to this surface stripe is a broad streak of internal
melanophores extending above and below the lateral
midline. New recruits develop a "sharknose"
appearance, where the tip of the snout extends
forward over the upper jaw.
|
|
|
|
| Elacatinus
lobeli transitional recruit |
| 8.5 mm SL |
| Utila, Honduras |
|
 |
| |
 |
|
|

|
|
|

|
|
|
|
|
|
|
|
|
| Diagnosis:
Fused pelvic fins and modal fin-ray counts of D-VII,12
A-11 and Pect-16-17 indicate some of the cleaner
gobies of the genus Elacatinus
(formerly considered Gobiosoma). The common
shallow-water cleaner goby in Puerto Rico and the
Lesser Antilles is E.
evelynae, a V-nosed species. |
|
| Analogues:
Larvae of the cleaner gobies are likely identical
(analogues discussed above under E.
illecebrosus), but recruits and juveniles
of E. evelynae are separated from the other
V-nosed species (E. genie and E. prochilos,
both also "sharknosed") by a broad frenum connecting
the snout to the upper lip. E. genie, a very
similar species from the Bahamas and the Cayman
Islands that shares the sharknose appearance is
distinguished by having the upper lip separated
from the snout by a deep groove. E. prochilos
has the upper-lip groove, a more Y-shaped mark on
the snout, and is not supposed to have the thin
pale dorsal midline stripe from the dorsal fin forward
(but whether this applies to new recruits is uncertain).
The morphological differences of the mouth and snout
may not be useful in the earliest recruit stages.
|
|
| Description:
Larvae of the cleaner gobies are likely
identical (described above under E.
illecebrosus). Transitional recruits develop
two wide dark stripes along the top of the head
(separated by only a thin clear line) that merge
to become a stripe along the base of the dorsal
fin fading out between the two dorsal fins. Below
the dark stripe is a prominent white stripe (yellow/blue
in life) that meets the stripe from the other side
in a distinct V-shape on the snout. A wide lateral
dark stripe starts at the tip of the upper jaw and
continues through the eye along the side of the
body just below the lateral midline to the caudal
fin, widening at the caudal-fin base and then extending
out along the central and lower fin rays. Beneath
this surface stripe is a broad dark stripe of internal
melanophores that extends above and below the lateral
midline. |
|
|
|
| Elacatinus
evelynae transitional recruits |
| 9.1 and 8.6 mm SL |
| St. Thomas, USVI, ST506 |
|
 |
| Elacatinus
evelynae transitional recruits |
| 9.0, 8.7 and 8.6 mm
SL |
| St. Thomas, USVI, ST506 |
|
 |
|
|
|
|
|
|
|
|
| Diagnosis:
Fused pelvic fins and modal fin-ray counts of D-VII,12
A-11 and Pect-16-17 indicate some of the cleaner
gobies of the genus Elacatinus
(formerly considered Gobiosoma). The endemic
Elacatinus phthirophagus
is the only cleaner goby present in Noronha in Brazil. |
|
| Analogues:
Larvae of the cleaner gobies are likely identical
(analogues discussed above under E.
illecebrosus), but recruits and juveniles
of E. phthirophagus are separated from other
bar-nosed species by having a mostly light area
between the eye and the mid-upper jaw, but with
a dark outline around the light bar. E. randalli
has the snout all light (the bar isn't outlined
by dark). E. cf xanthiprora usually also
has an all light snout; it can be dusky, but the
bar is not distinctly outlined as in E. phthirophagus.
The other bar-nosed species have a dark snout (E.
illecebrosus and E. xanthiprora).
Adults are also separated by color and head shape:
E. phthirophagus has a wide yellow lateral
stripe (the others have narrow yellow stripes),
while E. illecebrosus has an underslung upper
lip (not shared by the other bar-nosed species)
and E. cf xanthiprora has a white lateral
stripe becoming yellow near the head. |
|
| Description:
Larvae of the cleaner gobies are likely identical
(described above under E.
illecebrosus). Transitional recruits develop
two well-separated thin dark stripes along the top
of the head that merge to become a stripe along
the base of the dorsal fin fading out before the
caudal peduncle. The snout is mostly unpigmented,
but has two thin extensions of the dark stripes
from the top of the head and a short tenuous bar
along the midline. A lateral dark stripe develops
from the middle of the eye to the caudal fin widening
at the caudal-fin base and then extending out along
the central and lower fin rays. Beneath this surface
stripe is a broad dark stripe of internal melanophores.
A thin white (yellow in life) stripe then develops
within the pale band along the upper body. |
|
|
|
Elacatinus
phthirophagus
transitional recruit |
| 8.8 mm SL |
| Noronha, Brazil FN01 |
|
 |
| |
 |
| Elacatinus
phthirophagus |
| 27.0 mm SL |
| Noronha, Brazil FN01 |
|

|
|
|
|
 |
|
All contents © copyright 2006-2014
All rights reserved
www.coralreeffish.com by
Benjamin Victor
|
|
|
|
|
| |
|
| |