|
for pdf reprints, click on the title of the articles
below
|
 |
 |
Helmkampf, M, Coulmance, F, Heckwolf, MJ, Acero P, A, Balard, A, Bista, I, Dominguez, OD, Frandsen, PB, Torres-Oliva, M, Santaquiteria, A, Tavera, J, Victor, BC, Robertson, DR, Betancur-R, R, McMillan, WO and Puebla, O (2025) |
Science Advances, 11(30) eadt0973
https://doi.org/
10.1126/sciadv.adt0973 |
|
Radiation with reproductive isolation in the near-absence of phylogenetic signal |
 |
Data 2025
10 (7), 108
https://doi.org/
10.3390/data10070108 |
A DNA Barcode Dataset for the Aquatic Fauna of the Panama Canal: Novel Resources for Detecting Faunal Change in the Neotropics (2025)
|  |
|
Saltonstall, K, Collin, R, Aguilar, C, Alda, F, Baldrich-Mora, LM, Bravo, V, Castillo, MF, Castro, S, De Leon, LF, Diaz-Ferguson, E, Garces, HA, Gomez,E, Gonzalez, RG, Gonzalez-Torres, MA, Guzman, HM, Hiller, A, Ibanez, R, Jaramillo, C, Kaiser, KL, Kam, Y, Peralta, ML, Lopez, OG, Madrid Concepcion, ME, Miller, MJ, Ossa-Hernandez, N, Reina, RG, Robertson, DR, Romero-Gonzalez, TE, Sandoval, M, Sanjur, O, Schloder, C, Sharpe, AE, Sharpe, D, Siepmann, J, Strasiewsky, D, Torchin, ME, Tumbaco, M, Vargas, M, Venegas-Anaya, M, Victor, BC and Castellanos-Galindo, G (2025) |
 |
 |
Victor,
BC (2025) |
Journal of the Ocean Science Foundation
43: 12-38 |
|
Prionotus pictus, a new endemic species of searobin from the Galapagos Islands, Ecuador (Teleostei: Triglidae) |
 |
Journal of the Ocean Science Foundation
42: 7-14 |
Grove, JS and Victor, BC
(2025) |
 |
|
Has climate change driven the Galapagos Damselfish, Azurina eupalama, to extinction? |
 |
 |
Victor,
BC (2024)
|
In: Early Life History and Biology of Marine Fishes: Research inspired by the work of H Geoffrey Moser. J.M. Leis, W.Watson, B.C. Mundy & P. Konstantinidis (Eds.), NOAA Professional Paper NMFS 24: 273-286 |
|
Rapid long-distance multispecies transport of
shorefish larvae to the oceanic tropical eastern
Pacific, revealed by DNA barcodes and otolith
aging of larvae captured over the Galapagos Rift |
 |
Journal of the Ocean Science Foundation
41: 112-137 |
Robertson, DR, Dunlap-Smith, AG, Goolishian Hernandez, AM, Richter, L, Richter, S, Mitchell, S, Estape, CJ, Morgan Estape, A and Victor, BC (2024) |
 |
|
Additions to the marine fish fauna of the US Virgin Islands (2024) |
 |
 |
Victor,
BC, Grove, JS, Long, DL, Robertson, DR, Keith, I, Bensted-Smith, W and Salinas-de-Leon, P (2024) |
Journal of the Ocean Science Foundation
41: 54-111 |
|
List of Fishes of the Galapagos Archipelago, Ecuador (Version 2.0) |
 |
peerj 12.e16828
https://peerj.com/articles/
16828/ |
Victor, BC, Frable, BW and Ludt, WB
(2024) |
 |
|
Halichoeres sanchezi n. sp., a new wrasse from the Revillagigedo Archipelago of Mexico, tropical eastern Pacific Ocean (Teleostei: Labridae) |
 |
 |
 |
Randall, JE and Victor,
BC (2022)
Volume 4 Family Labridae |
South African Institute for Aquatic Biodiversity Special Publication, Makhanda, South Africa
Vol. 4: 166-265, plates 55-102 |
|
In: Heemstra, PC, Heemstra, E, Ebert, DA, Holleman, W & Randall, JE (eds.) Coastal fishes of the Western Indian Ocean |
 |
South African Institute for Aquatic Biodiversity Special Publication, Makhanda, South Africa
Vol. 4: 93-116, plates 25-32 |
Randall, JE and Victor,
BC (2022)
Volume 4 Family Pempheridae |
 |
|
In: Heemstra, PC, Heemstra, E, Ebert, DA, Holleman, W & Randall, JE (eds.) Coastal fishes of the Western Indian Ocean |
 |
 |
Grove, JS, Long, DL, Robertson, DR and Victor,
BC (2022) |
Journal of the Ocean Science Foundation
39: 14-22 |
|
List of Fishes of the Galapagos Archipelago, Ecuador |
 |
ZooKeys
1103: 79-122 |
Robertson, DR, Estape, CJ, Estape, AM, Richter, L, Pena, E and Victor,
BC (2022) |
 |
|
An updated, illustrated inventory of the marine fishes of the US Virgin Islands |
 |
 |
Allen, GR, Victor,
BC and Erdmann, MV (2021) |
Journal of the Ocean Science Foundation
38: 66-77 |
|
Lethrinus mitchelli, a new species of emperor fish (Teleostei: Lethrinidae) from Milne Bay Province, Papua New Guinea |
 |
Acta Ichthyologica Piscatoria
50(4): 501-510 |
Zajonz, U, Bogorodsky, SV and Victor,
BC (2020) |
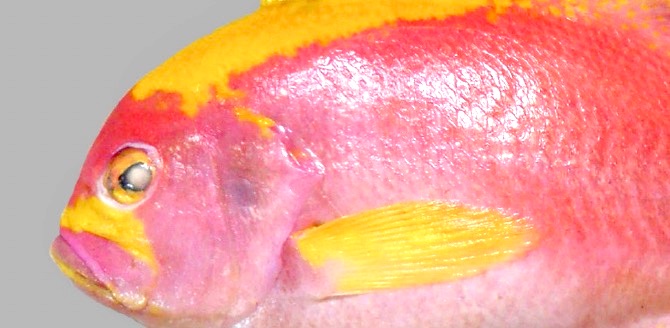 |
|
First record of Meganthias natalensis (Actinopterygii: Serranidae: Anthiadinae) from the Socotra Archipelago (north-western Indian Ocean), with notes on Odontanthias and Sacura |
 |
 |
Victor,
BC (2020) |
Journal of the Ocean Science Foundation
37: 1-122 |
|
Review of the glass blennies (Teleostei: Chaenopsidae: Emblemariopsis) with two new species from the Caribbean Sea |
 |
Journal
of the Ocean Science Foundation
36: 6-15 |
Victor,
BC, Teitelbaum, A and Randall, JE (2020) |
 |
|
Pseudanthias timanoa, a new fairy basslet from New Caledonia, South Pacific (Teleostei: Serranidae: Anthiadinae) |
 |
 |
Smith-Vaniz, WF, Victor,
BC and Allen, GR (2020) |
Journal of the Ocean Science Foundation
35: 118-128 |
|
Aggressive mimicry in Aspidontus and Plagiotremus (Pisces: Blenniidae): some mimetic phenotypes are not phylogenetically informative |
 |
Journal
of the Ocean Science Foundation
35: 76-85 |
Victor, BC and Ianniello, L (2020) |
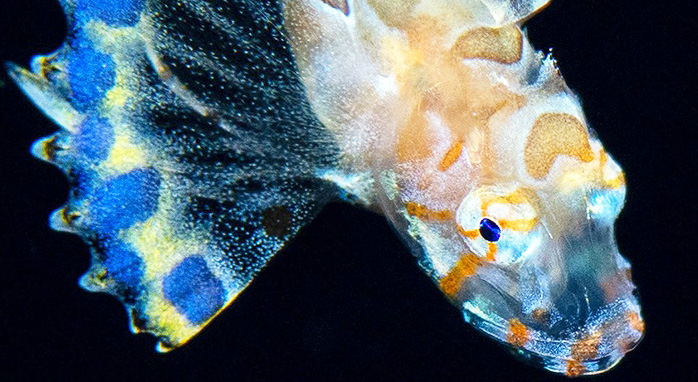 |
|
Prionotus murielae Mowbray, 1928 is the juvenile of the Bandtail Searobin Prionotus ophryas (Teleostei: Scorpaeniformes: Triglidae) |
 |
 |
Victor,
BC and Kumar, AB (2019) |
Journal of the Ocean Science Foundation
33: 70-78 |
|
Pteropsaron indicum, a new species of signalfish (Teleostei: Trichonotidae: Hemerocoetinae) with a micro-CT analysis of osteology |
 |
Journal
of the Ocean Science Foundation
32: 1-16 |
Victor,
BC (2019) |
 |
|
Enneanectes flavus, a new endemic species of triplefin
blenny from the southeastern Caribbean (Teleostei: Tripterygiidae) |
 |
 |
Victor,
BC (2018) |
Journal of the Ocean Science Foundation
31: 54-73 |
|
Starksia splendens, a new endemic labrisomid blenny from
the Cayman Islands (Teleostei: Labrisomidae) |
 |
Journal
of the Ocean Science Foundation
31: 8-17 |
Victor,
BC and Marks, KW (2018) |
 |
|
Hypoplectrus liberte, a new and endangered microendemic
hamlet from Haiti (Teleostei: Serranidae |
 |
 |
Victor,
BC and Krasovec, FH (2018) |
Journal of the Ocean Science Foundation
31: 1-7 |
|
Facultative cleaning behavior in a western Atlantic sponge goby,
Elacatinus xanthiprora (Teleostei: Gobiidae) |
 |
| PeerJ
6:e4328; DOI 10.7717/peerj.4328 |
Robertson,
DR, Dominguez-Dominguez, O, Victor, BC and Simoes, N. (2018) |
 |
|
An Indo-Pacific damselfish (Neopomacentrus cyanomos)
in the Gulf of Mexico: origin and mode of introduction |
 |
 |
Victor,
BC (2017) |
Journal of the Ocean Science Foundation
29: 11-31 |
|
Review of the Indo-Pacific Pseudojuloides cerasinus
species complex with a description of two new species
(Teleostei: Labridae) |
 |
Journal
of the Ocean Science Foundation
27: 48-73 |
Victor,
BC (2017) |
 |
|
The status of Enneanectes jordani and a new species of
triplefin blenny from the Greater Caribbean (Teleostei: Tripterygiidae) |
 |
 |
Victor,
BC (2016) |
Journal of the Ocean Science Foundation
23: 21-50 |
|
Two new species in the spike-fin fairy-wrasse species complex
(Teleostei: Labridae: Cirrhilabrus) from the Indian Ocean |
 |
Journal
of the Ocean Science Foundation
22: 10-27 |
Victor,
BC (2016) |
 |
|
Halichoeres gurrobyi, a new labrid fish (Teleostei: Labridae)
from Mauritius in the southwestern Indian Ocean, with a review
of the H. zeylonicus species complex |
 |
 |
Victor,
BC and Edward, JMB (2016) |
Journal of the Ocean Science Foundation
21: 58-70 |
|
Pseudojuloides labyrinthus, a new labrid fish (Teleostei:
Labridae) from the western Indian Ocean |
 |
Journal
of the Ocean Science Foundation
18: 1-77 |
Randall,
JE and Victor, BC (2015) |
 |
| Descriptions
of thirty-four new species of the fish genus Pempheris
(Perciformes: Pempheridae), with a key to the species of the
western Indian Ocean
|
 |
 |
Victor,
BC, Valdez-Moreno, M and Vásquez-Yeomans, L. (2015) |
DNA Barcodes
2015 (3): 85-93 |
|
Status of DNA Barcoding Coverage for the Tropical Western Atlantic
Shorefishes and Reef Fishes |
 |
Journal
of the Ocean Science Foundation
16: 1-55 |
Randall,
JE, Connell, A.D. and Victor, BC (2015) |
 |
| Review
of the labrid fishes of the Indo-Pacific Genus Pseudocoris,
with a description of two new species
|
 |
 |
Victor,
BC and Edward, JMB (2015) |
Journal of the Ocean Science Foundation
15: 41-52 |
|
Pseudojuloides zeus, a new deep-reef wrasse (Perciformes:
Labridae) from Micronesia in the western Pacific Ocean |
 |
|
Cambridge
University Press, Cambridge, United Kingdom
chapter 8: 76-87
email
for pdf copy
book
on Amazon
|
Victor,
BC (2015)
Chapter
8
|
 |
|
How
many coral reef fish species are there? Cryptic diversity
and the new molecular taxonomy
In:
Mora, C. (Ed.) Ecology of Fishes on Coral Reefs
|
 |
 |
Connell,
AD, Victor, BC and Randall, JE (2015) |
Journal of the Ocean Science Foundation
14: 49-56 |
|
A new species of Pseudojuloides (Perciformes: Labridae)
from the south-western Indian Ocean |
 |
Journal
of the Ocean Science Foundation
12: 61-83 |
Randall,
JE and Victor, BC (2014) |
 |
| Four
new fishes of the genus Pempheris (Perciformes: Pempheridae)
from the western Indian Ocean
|
 |
 |
Randall,
JE, Victor, BC, Alpermann, TJ, Bogorodsky, SV, Mal, AO, Satapoomin,
U and Bineesh, KK (2014) |
Zootaxa
3887:
377-392 |
|
Rebuttal to Koeda et al. (2014) on the Red Sea fishes
of the perciform genus Pempheris |
 |
| Bulletin
of Marine Science 90(1):533-549 |
Bernardi,
G, Ramon, ML, Alva-Campbell, Y, McCosker, JE, Bucciarelli, G,
Garske, LE, Victor, BC and Crane, NL (2014) |
 |
| Darwin's
fishes: phylogeography of Galapagos Islands reef fishes
|
 |
 |
Victor,
BC (2014) |
Journal of the Ocean Science Foundation
12: 25-60 |
|
Three new endemic cryptic species revealed by DNA barcoding
of the gobies of the Cayman Islands (Teleostei: Gobiidae) |
 |
Journal
of the
Ocean Science
Foundation
11: 1-12 |
Victor,
BC and Randall, JE (2014) |
 |
| Pseudojuloides
edwardi, n. sp. (Perciformes: Labridae): an example of evolution
of male-display phenotype outpacing divergence in mitochondrial
genotype
|
 |
 |
Randall,
JE and Victor, BC (2013) |
Journal of the Ocean Science Foundation
8: 44-61 |
| Bodianus
atrolumbus (Valenciennes 1839), a valid species of labrid
fish from the southwest Indian Ocean |
 |
Journal
of the
Ocean Science
Foundation
8: 30-43 |
Victor,
BC (2013) |
 |
| Scorpaena
wellingtoni n. sp., a new scorpionfish from the Galapagos
Islands (Scorpaeniformes: Scorpaenidae)
|
 |
 |
Victor,
BC (2013) |
Journal of the Ocean Science Foundation
7: 44-73 |
| The
Caribbean Roughhead Triplefin (Enneanectes boehlkei):
DNA barcoding reveals a complex of four West Indian sympatric
cryptic species (Teleostei: Blennioidei: Tripterygiidae) |
 |
Zootaxa
3669: 551-570 |
Victor,
BC, Alfaro, ME and Sorenson, L (2013) |
 |
| Rediscovery
of Sagittalarva inornata n. gen., n. comb. (Gilbert,
1890) (Perciformes: Labridae), a long-lost deepwater fish from
the eastern Pacific Ocean: a case study of a forensic approach
to taxonomy using DNA barcoding
|
 |
 |
Victor,
BC and Wellington, GM (2013) |
Journal of the Ocean Science Foundation
6: 19-32 |
| Citharichthys
darwini n. sp., a new endemic flatfish from the Galapagos
Archipelago (Teleostei: Pleuronectiformes: Paralichthyidae) |
 |
Journal
of the
Ocean Science
Foundation
5: 1-19 |
Victor,
BC (2012) |
 |
| Hypoplectrus
floridae n. sp. and Hypoplectrus ecosur n. sp., two
new Barred Hamlets from the Gulf of Mexico (Pisces: Serranidae):
more than 3% different in COI mtDNA sequence from the Caribbean
Hypoplectrus species flock
|
 |
 |
Baldwin,
CC, Castillo, CI, Weigt, LA and Victor, BC (2011) |
ZooKeys
79: 21-72 |
|
Seven new species within western Atlantic Starksia atlantica,
S. lepicoelia, and S. sluiteri (Teleostei, Labrisomidae),
with comments on congruence of DNA barcodes and species |
 |
Journal
of the
Ocean Science
Foundation
4: 1-29 |
Victor,
BC (2010) |
 |
| Emblemariopsis
carib and Emblemariopsis arawak, two new chaenopsid
blennies from the Caribbean Sea: DNA barcoding identifies males,
females, and juveniles and distinguishes sympatric cryptic species
|
 |
 |
Victor,
BC and Randall, JE (2010) |
Zoological
Studies
49(6):
865-871 |
|
Gramma dejongi, a new basslet (Perciformes: Grammatidae)
from Cuba, a sympatric sibling species of G. loreto |
 |
Journal
of the
Ocean Science Foundation
3: 1-16 |
Victor,
BC (2010) |
 |
| The
Redcheek
Paradox: the mismatch between genetic and phenotypic divergence
among deeply-divided mtDNA lineages in a coral-reef goby, with
the description of two new cryptic species from the Caribbean
Sea
|
 |
 |
Victor,
BC, Vasquez-Yeomans, L, Valdez-Moreno, M, Wilk, L, Jones, DL,
Lara, M, Caldow, C and Shivji, M (2010) |
Zootaxa
2346:
53-61 |
|
The larval, juvenile, and adult stages of the Caribbean goby,
Coryphopterus kuna (Teleostei: Gobiidae): a reef fish
with a pelagic larval duration longer than the post-settlement
lifespan |
 |
Zootaxa
2215: 24-36 |
Victor,
BC, Hanner, R, Shivji, M, Hyde, J and Caldow, C (2009) |
 |
| Identification
of the larval and juvenile stages of the Cubera Snapper, Lutjanus
cyanopterus, using DNA barcoding
|
 |
 |
Victor,
BC (2008) |
Journal of the Ocean Science Foundation
1: 1-19 |
| Redescription
of Coryphopterus tortugae (Jordan) and a new allied species
Coryphopterus bol (Perciformes: Gobiidae: Gobiinae) from
the tropical western Atlantic Ocean |
 |
Zootaxa
1526: 51-61 |
Victor,
BC (2007) |
 |
| Coryphopterus
kuna, a new goby (Perciformes: Gobiidae: Gobiinae) from
the western Caribbean, with the identification of the late larval
stage and an estimate of the pelagic larval duration |
 |
 |
Robertson,
DR, Karg, F, Leao de Moura, R, Victor, BC and Bernardi, G (2006) |
Molecular
Phylogenetics and Evolution
40: 795-807 |
| Mechanisms
of speciation and faunal enrichment in Atlantic parrotfishes |
 |
ASIH 2006
meeting abstracts |
Victor,
BC (2006) |
 |
| The
late-stage larvae of Caribbean gobies, eleotrids, and microdesmids:
identification guide and patterns of size and age at settlement |
 |
 |
Victor,
BC, Wellington, GM, Robertson, DR and Ruttenberg, BI (2001) |
Bulletin
of Marine Science
69(1): 279-288 |
| The
effect of the El Nino-Southern Oscillation event on the distribution
of reef-associated labrid fishes in the eastern Pacific Ocean |
 |
Proceedings of the Royal Society of
London, B.
268: 1931-1936 |
Riginos,
C and Victor, BC (2001) |
 |
| Larval
spatial distributions and other early life history characteristics
predict genetic differentiation in eastern Pacific blennioid
fishes |
 |
 |
Victor,
BC, Wellington, GM and Caldow, C (2001) |
Revista Biologia Tropical
49(1): 101-110 |
| A
review of the razorfishes (Perciformes:Labridae) of the eastern
Pacific Ocean |
 |
In: D. and P. Hoply,
J. Talemander, and
T. Done, eds.
Proc. of the 9th
Int. Coral Reef Symposium
Abstracts 2000: 10 |
Victor,
BC, Wellington, GM and Robertson, DR (2000) |
 |
| The
effect of El Nino on the distribution of reef-associated labrid
fishes in the eastern Pacific Ocean |
 |
 |
Victor,
BC and Wellington, GM (2000) |
Marine Ecology Progress Series
205: 241-248 |
| Endemism
and the pelagic larval duration of reef fishes in the eastern
Pacific Ocean |
 |
Copeia
1992
4: 1053-1059 |
Wellington,
GM (1992) (collaboration) |
 |
| Xyrichtys
victori, a new species of razorfish from the Galapagos Islands
(Teleostei: Labridae) |
 |
 |
Wellington,
GM and Victor, BC (1992) |
Marine
Biology
113: 491-498 |
| Regional
differences in the planktonic larval duration of reef fishes
in the eastern Pacific Ocean |
 |
In: Sale, P, ed.
The ecology of fishes on coral reefs.
Orlando, Florida,
Academic Press
1991: 231-60 |
Victor,
BC (1991) |
 |
| Settlement
strategies and biogeography of reef fishes |
 |
 |
Wellington,
GM and Victor, BC (1989) |
Marine
Biology
101: 557-567 |
| Planktonic
larval duration of one hundred species of Pacific and Atlantic
damselfishes (Pomacentridae) |
 |
Ecology
69: 370-381 |
Robertson,
DR, Green, DG and Victor, BC (1988) |
 |
| Temporal
coupling of production and recruitment of larvae of a Caribbean
reef fish |
 |
 |
Wellington,
GM and Victor, BC (1988) |
American
Naturalist
131: 588-601 |
| Variation
in components of reproductive success in an undersaturated population
of a coral reef damselfish: a field perspective |
 |
Bulletin of Marine Science
40: 152-160 |
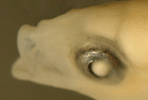
Victor,
BC (1987) |
 |
| The
mating system of the Caribbean rosy razorfish, Xyrichtys
martinicensis |
 |
 |
Victor,
BC (1987) |
Marine
Biology
95: 145-152 |
| Growth,
dispersal, and identification of planktonic labrid and pomacentrid
reef-fish larvae in the eastern Pacific Ocean |
 |
Canadian
Journal
of Fisheries and
Aquatic Sciences
43: 1208-1213 |
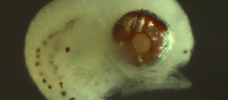
Victor,
BC (1986) |
 |
|
Delayed metamorphosis with reduced larval growth in a coral
reef fish, Thalassoma bifasciatum |
 |
 |
Victor,
BC (1986) |
Marine
Biology
90: 317-326 |
| The
duration of the planktonic larval stage of one hundred species
of Pacific and Atlantic wrasses (Family Labridae) |
 |
Ecological
Monographs
56: 145-160 |
Victor,
BC (1986) |
 |
| Larval
settlement and juvenile mortality in a recruitment-limited coral
reef fish population |
 |
 |
Wellington,
GM and Victor, BC (1985) |
Oecologia
68: 15-19 |
| El
Nino mass coral mortality: a test of resource limitation in
a coral reef damselfish population |
 |
Limnology
and Oceanography
29: 1116-1119 |
Victor,
BC (1984) |
 |
| Coral
reef fish larvae: patch size estimation and mixing in the plankton |
 |
 |
Victor,
BC (1983) |
In:
Reaka, ML, ed.
The ecology of deep
and shallow reefs.
US Dept of Commerce, DC
1983: 47-51 |
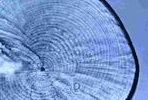
| Settlement
and larval metamorphosis produce distinct marks on the otoliths
of the slippery di ck Halichoeres bivittatus |
 |
Science
219:419-420 |
Victor,
BC (1983) |
 |
| Recruitment
and population dynamics of a coral reef fish |
 |
 |
Victor,
BC (1982) |
Marine
Biology
71: 203-208 |
| Daily
otolith increments and recruitment in two coral reef wrasses,
Thalassoma bifasciatum and Halichoeres bivittatus |
 |
Canadian
Journal
of Zoology
60: 2543-2550 |
Victor,
BC and Brothers, EB (1982) |
 |
| Age
and growth of the fallfish Semotilus corporalis with
daily otolith increments as a method of annulus verification |
 |