|
|
|
| The
serranids, or groupers and seabasses,
are ubiquitous predators on Caribbean
coral reefs and come in all shapes and
sizes. Groupers are, or more accurately,
were the basis of large and important
fisheries throughout the region, but
have become rare on fished reefs and
some species are even endangered. This
large and diverse family is composed
of several clearly-defined subfamilies.
Those subfamilies with Caribbean reef-associated
representatives include the conspicuous
groupers (Epinephelinae),
the numerous small seabasses and hamlets
(Serraninae),
the deep-water basslets (Liopropominae),
the soapfishes (Grammistinae),
and the small reef-bass Pseudogramma
gregoryi (also can be considered
either a Grammistinine or a Pseudogrammatinine).
There are many serranids in the region
found only in deep water and not associated
with coral reefs, nevertheless they
are included in this section for completeness. |
| |
| Larval
serranids can be recognized by their
relatively wide body, particularly large
terminal mouth, large round eye, continuous
spinous and soft dorsal fins with stout,
sometimes serrated, dorsal-fin spines,
and three stout spines in a short anal
fin (note these characters are not all
shared by the Grammistinae).
Unfortunately, these characters are
shared with many other percoid fishes
and some serranid fin-ray counts can
overlap with the abundant snappers (Lutjanidae)
and grunts (Haemulidae).
Within each of these families there
is variation in the larval body form
and meristics and often marked ontogenetic
changes as well, leading to some similarity
in appearance and the distinction sometimes
can be difficult. |
| |
| In
the earlier stages, the larvae of the
three families are quite similar and
separation is a problem. The serranids
and lutjanids
even share their basic marking patterns,
with melanophores on the spinous dorsal-fin
membranes and on the anal fin base and
caudal peduncle. A useful distinction
is that serranids typically have a melanophore
at the angle of the jaw and melanophores
on the pectoral-fin rays, which, if
present, immediately separate them from
the other families. Lutjanids
have a post-cleithral spine, not present
in the other families, but it is not
easy to see. In general, the three families
have a different look as later larvae:
serranids generally have large jaws
with a sharp jawline (essentially a
much wider gape than the others) and
a particularly wide maxilla, snappers
have less prominent jaw lines and an
enlarging non-serrated preopercular
spine, while grunts
typically have smaller larvae with shorter
dorsal-fin spines, and, importantly,
the third anal-fin spine develops as
a segmented ray in larvae and small
juveniles. |
| |
| Although
serranid larvae share the basic features
listed above, the size at settlement
varies widely within the family: hamlets
and basslets settle very small, from
5-10 mm SL, while some epinephelines
settle particularly large, sometimes
reaching up to two inches in length
while still pelagic. |
|
|
|
|
|
| |
|
|
|
| This
subfamily comprises the large commercially-important
groupers of the region. There are several
epinepheline genera with a single species
in the region (one with two) and then two
large genera, the Epinephelus
and the Mycteroperca.
The phylogenetics of the Serranidae have been
recently examined by Craig and Hastings (2007),
and they find that the regional Mycteroperca
do form a distinct clade, but the
regional Epinephelus
split into three clades: the true Epinephelus
including the common shallow-water species,
the deep-water set of species (or Hyporthodus),
and the deep-water E. drummondhayi
(or Triso). Interestingly, Paranthias
furcifer falls within the Cephalopholis
clade, despite its derived form and non-benthic
habits. This conclusion is reinforced by the
report of hybrids between the two genera from
both Noronha and Bermuda. |
|
| The
basic body form and appearance of many groupers
are the same and they are difficult to distinguish
in the field. The two large genera are most
easily separated by the anal-fin ray count:
only eight soft rays (occasionally nine) in
the Epinephelus
and usually 11 or more (rarely 10)
in the Mycteroperca
(all have 11 dorsal-fin spines and 3 anal-fin
spines, except E. nigritus with ten).
Two small reef groupers, the Graysby and the
Coney, belong to Cephalopholis, easily
separated by having only nine dorsal-fin spines.
The remaining regional epinephelines comprise
Paranthias furcifer (with D-IX,18-19
A-III,9), Dermatolepis inermis (with
D-XI,18-20 A-III,9), Alphestes afer
(with D-XI,18-19 A-III,9), and the deep-water
Gonioplectrus hispanus (with D-VIII,13
A-III,7). |
|
| Fin-ray
counts can identify most Caribbean epinepheline
larvae to genus relatively easily. However,
within genera there is a broad overlap of
fin-ray counts and little variation in body
form, making DNA-sequence analyses critical
to differentiating the larval groupers. |
| |
|
|
|
|
|
|
|
|
|
|
|
|
| Diagnosis:
Modal fin-ray counts of |
|
| A |
|
| Description:
|
|
|
|
|
|
|
|
|
|
|
|
| Diagnosis:
Modal fin-ray counts of |
|
| A |
|
| Description:
|
|
|
|
|
|
|
|
| |
|
|
|
| Distinguishing
this large group of small serranid fishes
from other serranid subfamilies can be subtle
and dorsal-fin spine and anal-fin soft-ray
counts are often necessary. All of the regional
serranines have ten dorsal-fin spines while,
serendipitously, the other regional serranids
have nine or fewer or eleven or more (except
E. nigritus). In addition, the serranines
(except for the deep-water anthiines) have
seven anal-fin soft rays and non-serrated
fin spines, while the remaining subfamilies
of Serranidae have eight or more anal-fin
soft rays and sometimes serrated fin spines.
Some larval snappers (Lutjanidae)
have a similar general appearance but have
eight or more anal-fin soft rays (and a prominent
preopercular spine). The larval grunts (Haemulidae)
have twelve or more dorsal-fin spines, are
generally narrower-bodied, and their third
anal-fin spine is a segmented ray in the larval
and early juvenile stages. There is certainly
some overlap in body shape between the narrower-bodied
serranines (such as Diplectrum)
and the haemulids,
in which case fin-ray characters are useful. |
|
| The
deep-water anthiine serranines are sometimes
raised to their own subfamily (Anthiinae)
and they are the only serranines to have some
species with a mode of eight anal-fin soft
rays. |
| |
| Dorsal-fin
soft-ray counts separate the genera:
There are several reef-associated Caribbean
serranine genera, generally separating into
two groups by dorsal-fin soft-ray counts:
the numerous hamlets of Hypoplectrus
with D-X,15 and the large genus of basslets
of Serranus,
typically with D-X,12. The deep-water serranines
have 10 or 11 or 13-16. |
| |
| Shallow-water
Serranus
species separate out slightly by modal pectoral
fin-ray counts (but the ranges overlap extensively):
a group with 14 comprising S.
baldwini (13-15), S.
tigrinus (14), and S.
tortugarum (13-15); then S.
tabacarius with 15 (sometimes 14);
S. subligarius
with 16 (14-17, a northern species), and S.
flaviventris with 16 (or 17, a southern
species). There are a number of deeper-water
species as well that broadly overlap these
counts: S. annularis
with 13 (or 14), S.
chionaraia with 14 (or 13), S.
luciopercanus with 14, S.
maytagi with 15-16, S.
notospilus and S.
phoebe with 15-16 (14-17) and S.
atrobranchus with 16 (15-17). |
| |
| The
remaining shallow-water serranine genera have
sometimes overlapping fin-ray counts with
the Serranus-
three Diplectrum
species:
D. bivittatum (X,12 Pect-15-16,
occ. 14), D.
formosum (X,12 Pect-16-17, up to 18),
and D. radiale
(X,12 Pect-16-18, mode 17, from the S. Caribbean);
Paralabrax dewegeri (X,13-14 Pect-17);
Serraniculus pumilio (X,10-11,
Pect-14-15); and Schultzea
beta (X,11-12 Pect-16). |
| |
| Two
deep-water serranines have fewer dorsal-fin
soft rays than the others: Parasphyraenops
atrimanus (X,10 III,6 Pect-17) and
P. incisus
(X,10 III,7 Pect-17). The Centropristis
species, from more temperate US waters only,
also can have fewer, with 11 (one with 12)
dorsal-fin soft rays and A-III,7 and comprise
C. fuscula
(with D-X,12), C.
ocyurus (Pect-17), C.
philadelphica (Pect-18), and C.
striata (Pect 16-19). |
| |
| The
remaining deep-water taxa tend to have 13
or more dorsal-fin soft rays and include Bullsichthys
caribbaeus (X,13-14 III,7 Pect-14-15)
and the anthiines. Anthiines comprise four
Anthias
species, all with D-X,14-15 Pect-18-21: i.e.
Anthias tenuis (III,8), A.
nicholsi (III,7), A.
woodsi (X,14, III,7), and A.
asperilinguis (X,15, III,7); Hemanthias
vivanus (X,14, III,8 Pect-18-19), H.
aureorubens (X,13-16,usually 15, III,8
Pect-16-17), and H.
leptus (X,14 III,8); Pronotogrammus
martinicensis (X,15 (13-16) III,7-8,
Pect-16-18), and, finally, Plectranthias
garrupellus with the unusual fin-ray
count of X,16 III,7 and Pect-13. Note that
some of these latter species with eight anal-fin
rays have identical fin-ray counts to some
lutjanid
snappers. |
|
|
|
|
|
|
|
|
|
|
Diagnosis:
Modal fin-ray counts of D-X,15 A-III,7
Pect-14 indicate the hamlets of Hypoplectrus.
There is a "species flock" of numerous color
variations of these fishes in the Caribbean,
many of which can hybridize and the small
juveniles are presumably indistinguishable.
Variant larvae with slightly different melanophore
patterns may either represent these different
"morphospecies" or individual
variation (likely the latter). DNA analysis
may not be diagnostic, since it has been
difficult to find consistent sequence divergence
between color morphs (Puebla
et al. 2007). The Caribbean morphospecies
comprise H.
aberrans, H. chlorurus, H. gemma, H. gummigutta,
H. indigo, H. guttavarius, H. nigricans,
H. providencia, H. puella, and
H. unicolor. The median-fin ray count
can be shared with some deep-water Caribbean
serranids (Anthias
nicholsi and A.
asperilinguis, some
Hemanthias aureorubens, and Pronotogrammus
martinicensis), but the latter species
have more pectoral-fin rays (16 or more).
(DNA)
|
|
| Analogues:
|
|
| Description:
Body thin and moderately wide with a large
round eye and very large terminal mouth. Pectoral
and pelvic fins medium length, reaching much
of the way to the vent, dorsal-fin base long
and anal-fin base short, caudal peduncle moderately
wide and short. The typical complement of
melanophores on the head consists of one at
the angle of the jaw and a sparse scattering
on the top of the head. On the body there
is one at the dorsal midline just forward
of the first dorsal spine and then two large
melanophores on the ventral midline of the
caudal peduncle; one just behind the last
anal-fin ray and one just before the first
procurrent caudal-fin ray (the latter are
often persistent through transition in serranines).
Melanophores on the fins are prominent: the
full complement consists of a patch on the
membranes of the third to fifth dorsal-fin
spines, several near the base of the first
three anal-fin soft rays, extensively lining
the membranes of the pectoral and pelvic fins
and finally one at the base of the lower central
caudal-fin rays. Internal melanophores are
present around the sacculus and along the
dorsal surface of the swim bladdder and the
peritoneum extending to the gut near the vent.
Varying patterns are common: earlier-stage
larvae can be missing the melanophores on
top of the head and/or the entire anal-fin,
caudal peduncle, and caudal fin set of melanophores.
Larvae approaching transition progressively
lose the markings on their pectoral and pelvic
fin rays and some larvae also develop a melanophore
on the dorsal midline of the caudal peduncle
(the saddle characteristic of juvenile hamlets).
One or both of the ventral caudal peduncle
spots occasionally are missing and sometimes
the anal fin has additional melanophores (sometimes
a full row) just distal to the base of the
rays. A variety of additional melanophores
occur in some individuals: just forward of
the nasal bones, along the ventral aspect
of the lower jaw, an additional melanophore
on the caudal peduncle after the last anal-fin
ray or a second spot on the base of the caudal-fin
rays (usually on the upper central caudal-fin
rays), or a few scattered on the caudal-fin
rays. Pre-transitional larvae have a somewhat-narrowed
vertical oval eye becoming fully round as
transition approaches. Transitional larvae
develop a fine scattering of discrete small
surface melanophores, dense towards the anterior
and fading towards the tail and the larval
melanophores progressively disappear (usually
starting with those on the pectoral-fin rays). |
|
|
|
| Hypoplectrus
sp. larva |
| 7.2 mm SL |
| San Blas, Panama,
SB86-1008 |
|
 |
| |
 |
| |
 |
| Hypoplectrus
sp. larva |
| 6.6 mm SL |
| slightly vertically
narrowed eye |
| San Blas, Panama,
SB87-222 |
|
 |
| Hypoplectrus
sp. early transitional larva |
| 7.3 mm SL |
| losing pectoral
fin melanophores |
| San Blas, Panama,
SB86-1004 |
|
 |
| |
 |
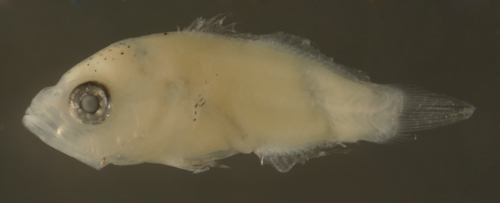
| Hypoplectrus
sp. larva |
| 6.2 mm SL |
variant pattern,
row of melanophores
on all anal-fin ray membranes and a
caudal peduncle spot above midline |
| San Blas, Panama,
SB83-179 |
|
 |
| |
 |
| Hypoplectrus
sp. larva |
| 7.0 mm SL |
variant pattern,
with caudal peduncle
spot on dorsal aspect |
| San Blas, Panama,
SB86-425 |
|
 |
| Hypoplectrus
sp. early transitional larva |
| 7.0 mm SL |
| variant pattern,
with two caudal fin spots |
| San Blas, Panama,
SB86-623 |
|
 |
| Hypoplectrus
sp. transitional larva |
| 7.1 mm SL |
variant pattern,
with three spots along
ventral aspect of caudal peduncle |
| San Blas, Panama,
SB83-156 |
|
 |
| |
 |
| Hypoplectrus
sp. transitional larva |
| 6.7 mm SL |
| San Blas, Panama,
SB83-156 |
|
 |
| |
 |
|
|
|
|
|
|
|
|
| There
are numerous species in this genus and DNA-sequence
analyses indicate that it is likely that the
genus is polyphyletic (M. Craig). Nevertheless,
many of these small basslets are very similar
in general appearance and overlap in meristics,
making it a difficult group for species identifications.
Most of the regional species share the median-fin
ray formula of D-X,12 A-III,7. DNA sequencing
is likely necessary to separate larvae of
some of the species, however identifications
can be narrowed down by pectoral-fin ray counts
and subtle differences in markings. |
| |
| Unlike
many other reef fishes, the Serranus
species lose their larval melanophore pattern
rapidly at transition and develop transitional
melanophores, often in a completely different
pattern. The distinction in melanophore size
is not as obvious as in many other fishes,
and the transitional melanophores can often
look the same as the larval melanophores they
are replacing. This is especially true on
the head and on the fin-ray membranes. Patches
of fin-membrane melanophores disappear and
new patches can arise on different parts of
the fin. In general, the transitional melanophores
on the body are smalller and more finely scattered
than the large, often single, larval melanophores.
Like the Hypoplectrus,
larvae of Serranus often show less
than their full complement of larval melanophores,
and occasional individuals have extra spots.
In addition, transitional larvae lose their
larval melanophores in no consistent order,
leading to a spectrum of spot patterns. This
variation makes identification more complicated
since variants are to be expected. |
| |
|
Species of Serranus
are listed in order of increasing pectoral-fin
ray counts
|
|
|
|
|
|
| |
|
|
|
|
Diagnosis:
Modal fin-ray counts of D-X,12 A-III,7 with
14 pectoral-fin rays indicate a subset of
Serranus
species. Shallow-water species with this
modal fin-ray count comprise S.
tigrinus, S.
baldwini, and S.
tortugarum (deep-water species comprise
S. chionaraia
and S. luciopercanus).
(ML)
|
|
| Analogues:
Larval S. tigrinus
can be separated from larval S.
baldwini by having melanophores at
the base of one or a few upper and lower caudal-fin
segmented rays. The eye of larval S.
tigrinus can be smaller than in its
congeners, with more than one eye-width into
the snout vs. less in the other species (note
this difference disappears in juveniles).
Larval S.
tortugarum and S.
tabacarius do not have the internal
melanophores on the mid-body or under the
last dorsal-fin spine and have melanophores
on the proximal portions of the middle spinous-fin
membranes (not the distal third). |
|
| Transitional
S. tigrinus
can be distinguished by having three head
stripes extending rearward from the upper
eye vs. two in S.
baldwini and none in S.
tortugarum and S.
tabacarius (the latter two species
also do not have the cheek stripe). The transitional
melanophores forming on the body at the base
of the fourth through sixth dorsal-fin spines
consist of a single long patch, while in S.
baldwini the melanophores split into
two patches (usually under the base of the
fourth and sixth, sparing the fifth). On S.
tortugarum and S.
tabacarius the melanophores concentrate
on the proximal fin membranes, not initially
forming patches on the adjacent body. |
|
|
Description:
Body thin and moderately wide with a round
eye and a large terminal mouth. Pectoral
and pelvic fins long, reaching just past
the vent, dorsal-fin base long and anal-fin
base short, caudal peduncle relatively wide
and short. The third spinous-dorsal-fin
membrane is greatly extended, up to 3 times
the length of the next membrane. On the
head there is a melanophore at the angle
of the jaw. On the body, melanophores are
mainly along the ventral midline of the
caudal peduncle, typically two or three:
the largest just after the last anal-fin
element and one or two just before the procurrent
caudal-fin rays (occasionally one or two
more in between). Some larvae have a melanophore
on the dorsal midline of the caudal peduncle
just after the last dorsal-fin element.
Two large deep internal melanophores are
present, one in the mid-upper body below
the mid-soft dorsal fin and one in the body
just below the base of the last dorsal-fin
spine (not always obvious in thick-bodied
preserved larvae). Internal melanophores
line the dorsal aspect of the swim bladder
and posterior peritoneum. The fin membranes
are well-marked, with the standard serranid
heavy speckling on the pelvic-fin membranes
and on the outer half of the pectoral-fin
membranes. There are melanophores along
the distal third of the spinous-dorsal-fin
membranes and lining the tips of the upper
and lower lobes of the caudal fin and at
the tips of all of the anal fin-ray membranes.
Some individuals may have them at the tips
of some of the dorsal soft-fin membranes
as well. There are also one or a few melanophores
near the base of the third anal-fin spine
and/or first soft ray. Notably there are
melanophores at the base of one (or a few)
of the mid-upper as well as mid-lower segmented
caudal-fin rays.
|
|
| Transitional
larvae develop three stripes radiating back
from the upper eye and an oblique stripe across
the cheek. As the markings increase the upper
two eye stripes merge and a reticulated pattern
of patches and lines develops on the anterior
upper body and near the base of the dorsal
fin; notably the patch near the base of the
fourth through sixth dorsal-fin spines is
not divided into two spots. The larval melanophores
rapidly fade away as the transitional surface
markings progress rearward, a stripe forms
from the eye to the tip of snout, and the
iris develops a clock-face-like set of surface
black patches. |
|
|
| Serranus
tigrinus early transitional
larva |
| 9.8 mm SL |
| Colon, Panama
N762a |
|
 |
|
|
 |
| Serranus
tigrinus transitional larva |
| 10.0 mm SL |
| Glover's Reef,
Belize |
|
 |
|
|
 |
|
|
 |
| Serranus
tigrinus transitional recruit |
| 10.7 mm SL |
| La Parguera,
Puerto Rico, PR784a |
|
 |
| Serranus
tigrinus transitional recruit |
| 11.0 mm SL |
| Colon, Panama,
N7531a |
|
 |
|
|
|
|
| |
|
|
|
|
Diagnosis:
Modal fin-ray counts of D-X,12 A-III,7 with
14 pectoral-fin rays indicate a subset of
Serranus
species. Shallow-water species with this
modal fin-ray count comprise S.
baldwini, S.
tortugarum, and S.
tigrinus (deep-water species comprise
S. chionaraia
and S. luciopercanus).
|
|
|
Analogues:
Larval S. baldwini
can be separated from larval S.
tigrinus by having no melanophores
at the base of the caudal-fin segmented
rays. Larval S.
tortugarum and S.
tabacarius do not have the internal
melanophores on the mid-body or under the
last dorsal-fin spine and have melanophores
on the proximal portions of the middle spinous-fin
membranes (not the distal third).
|
|
| Transitional
S. baldwini
can be distinguished by having two head stripes
extending rearward from the upper eye vs.
three in S.
tigrinus and none in S.
tortugarum and S.
tabacarius (the latter two species
also do not have the cheek stripe). The transitional
melanophores forming on the body at the base
of the fourth through sixth dorsal-fin spines
split into two patches (usually under the
base of the fourth and sixth, sparing the
fifth), while in S.
tigrinus the melanophores consist
of a single patch. On S.
tortugarum and S.
tabacarius the melanophores concentrate
on the proximal half of the fin membranes,
not initially forming patches on the body
below the fin. |
|
|
Description:
|
|
|
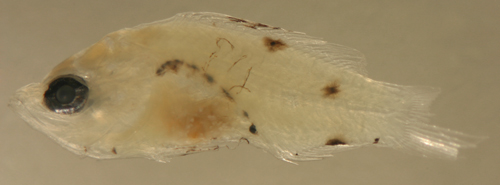
| Serranus
baldwini ? larva |
| 8.2 mm SL, pect.
14 |
| San Blas, Panama,
SB86-927 |
|
 |
|
|
|
|
| |
|
|
|
| Diagnosis:
Modal fin-ray counts of D-X,12 A-III,7 with
14 pectoral-fin rays indicate a subset of
Serranus
species. Shallow-water species with this modal
fin-ray count comprise
S. baldwini, S.
tortugarum, and S.
tigrinus (deep-water species comprise
S. chionaraia
and S. luciopercanus). |
|
| Analogues:
Larval S. tortugarum
have no melanophores on the caudal fin, while
larval S.
tabacarius have a melanophore at the
base of the upper segmented caudal-fin rays.
Larval S.
baldwini and S.
tigrinus differ by having internal
melanophores at the mid-body and under the
last dorsal-fin spine and the melanophores
along their dorsal-fin membranes are on the
distal third (not proximal). Larval Diplectrum
bivittatum differ by having a full
row of melanophores along the anal-fin base
and the row continues along the ventral midline
of the caudal peduncle. |
|
| Transitional
S. tortugarum
can be distinguished from S.
tabacarius by developing two distinct
patches of melanophores along the spinous-dorsal-fin
membranes vs. one. They have no obvious stripes
radiating from the eye as are found on transitional
S.
tigrinus and S.
baldwini. Transitional Diplectrum
bivittatum differ by having a prominent
mid-lateral body stripe and a distinctly narrower
body. |
|
| Description:
Body thin and moderately wide with a large
round eye and a large terminal mouth. Pectoral
and pelvic fins medium length, reaching much
of the way to the vent, dorsal-fin base long
and anal-fin base short, caudal peduncle moderately
wide and short. Dorsal and anal-fin spines
stout and non-serrated, three anal-fin spines.
Pre-transitional larvae are lightly marked,
with melanophores on the head only at the
angle of the jaw. On the body there is a large
melanophore at the ventral midline of the
caudal peduncle just after the last anal-fin
ray and a deep one before the start of the
procurrent caudal-fin rays. On the dorsal
fin there are patches of melanophores on the
membranes between the third and sixth spines
(mid-length on the third, proximal on the
fourth and fifth). Internal melanophores line
the dorsal surface of the swim bladder and
peritoneum extending to the gut near the vent.
Transitional larvae develop melanophores in
a large patch on top of the head and in a
row along the dorsal midline, starting with
four patches: the first just forward of the
dorsal-fin origin, the largest on and below
the fin membranes at the fourth and fifth
dorsal-fin spines, then on and below the last
three dorsal-fin spines, then on and below
the base of the third through fifth soft dorsal-fin
rays. At the same time the larval melanophores
are lost from the pectoral and pelvic fin
membranes and then from the body, with the
last persisting larval melanophore the one
just before the lower procurrent caudal-fin
rays. A broad irregular stripe of fine melanophores
extends rearward from the upper edge of the
operculum. Melanophores appear on the iris
at about 2, 3, 5, 8, and 11 o'clock. |
|
|
|
| Serranus
tortugarum early transitional
larva |
| 8.1 mm SL |
| 13 pectoral-fin
rays |
| San Blas, Panama,
SB86-101 |
|
 |
| |
 |
| Serranus
tortugarum transitional larva |
| 7.8 mm SL |
| San Blas, Panama,
SB86-623 |
|
 |
| |
 |
| Serranus
tortugarum late transitional
larva |
| 8.0 mm SL |
| San Blas, Panama,
SB86-1004 |
|
 |
| |
 |
| |
 |
| Serranus
tortugarum transitional recruit |
| 9.9 mm SL |
| San Blas, Panama,
SB82- |
|
 |
| |
 |
|
|
|
|
| |
|
|
|
|
Diagnosis:
Modal fin-ray counts of D-X,12 A-III,7 with
15 pectoral-fin rays indicate several Serranus
species and
Diplectrum bivittatum. Serranus
tabacarius is the only shallow-water
species with this modal fin-ray count (deep-water
species comprise S.
maytagi, S. notospilus, and S.
phoebe).
|
|
| Analogues:
Larval S. tabacarius
have a melanophore at the base of the upper
segmented caudal-fin rays while larval S.
tortugarum have no melanophores on
the caudal fin. Larval S.
baldwini and S.
tigrinus differ by having internal
melanophores at the mid-body and under the
last dorsal-fin spine and the melanophores
along their dorsal-fin membranes are on the
distal third (not proximal). Larval Diplectrum
bivittatum differ by having a full
row of melanophores along the anal-fin base
and the row continues along the ventral midline
of the caudal peduncle. |
|
|
Transitional S.
tabacarius can be distinguished from
S.
tortugarum by developing a single
large patch of melanophores along the spinous
portion of the dorsal-fin membranes vs. two.
They have no obvious stripes radiating from
the eye as are found on transitional S.
tigrinus and S.
baldwini. Transitional Diplectrum
bivittatum differ by having a prominent
mid-lateral body stripe and a distinctly narrower
body. |
|
|
Description:
Body thin and moderately wide with a round
eye and a very large terminal mouth. Pectoral
and pelvic fins long, reaching just past
the vent, dorsal-fin base long and anal-fin
base short, caudal peduncle relatively short
and not wide. (The third spinous-dorsal-fin
membrane is greatly extended, up to 3 times
the length of the next membrane)?. On the
head there is a melanophore at the angle
of the jaw. Later larvae can have additional
spots on the head, just above and behind
the upper eye, a spot over each lobe of
the brain, and a small midline patch just
behind the brain. On the body, there are
melanophores only along the ventral midline
of the caudal peduncle, one just after the
last anal-fin element and one or two just
before the procurrent caudal-fin rays. Internal
melanophores line the dorsal aspect of the
swim bladder and posterior peritoneum (no
internal melanophores in the mid-body or
tail). There is the standard serranid speckling
on the pelvic-fin membranes and on the outer
half of the pectoral-fin membranes. There
are melanophores along the membranes of
the posterior spinous dorsal fin (sixth
to ninth). On the base of the anal fin there
are one or two melanophores, usually on
the first soft ray and often the third or
fourth. Notably there is a melanophore at
the base of one (or a few) of the mid-upper
segmented caudal-fin rays.
|
|
|
| Serranus
tabacarius larva |
| 6.5 mm SL |
| San Blas, Panama,
SB84-519 |
|
 |
|
|
 |
|
|
|
|
| |
|
|
|
|
Diagnosis: Modal
fin-ray counts of D-X,12 A-III,7 with 15,
and often 16, pectoral-fin rays includes a
wide group of serranines including Diplectrum
in shallow waters and Serranus
atrobranchus, S. maytagi, S. notospilus,
and S. phoebe
along with Schultzea
beta, all in deeper waters. Shallow-water
Serranus
species with the high pectoral-fin ray count
include S. flaviventrus
with 16-17 and S.
subligarius (only from Florida and
GOM) with 15-17. Recruits and juveniles with
prominent lateral stripes indicate Diplectrum.
The Diplectrum
species are separated only slightly by modal
pectoral fin-ray counts (and scale counts):
15-16 in D. bivittatum
(54-75 lateral-line scales) and 16-17 in D.
formosum (46-55). D.
formosum occurs in US waters and from
Colombia to Brazil. The remaining species
in the genus, D.
radiale, has a mode of 17 pectoral-fin
rays and is found in the S. Caribbean only.
D. bivittatum
is the only species widespread in the region. |
|
| Analogues:
|
|
| Description:
Body thick and relatively narrow with a large
round eye and large terminal mouth. Pectoral
and pelvic fins medium length, reaching more
than half-way to the vent, dorsal-fin base
long and anal-fin base short, caudal peduncle
relatively narrow and short. Melanophores
typically at the angle of the jaw, a cluster
on the top of the head, and two stripes of
small melanophores, one along the upper body
only below the spinous dorsal-fin and one
full-length along the lateral midline. There
is a row of melanophores along the base of
the anal-fin soft rays, one per ray, and several
along the ventral midline of the caudal peduncle
ending before the start of the procurrent
caudal-fin rays. On the fins, melanophores
concentrate on the distal membranes between
the dorsal-fin spines and extensively speckled
along the pectoral and pelvic fin ray membranes.
There are often a few melanophores between
some of the anal-fin rays. On the caudal fin,
there are melanophores at the base of several
of the central lower segmented fin rays and
a larger patch at the base of the first two
or three upper segmented caudal-fin rays that
presents as a slight upward curve of the main
body mid-lateral stripe. Internal melanophores
are present along the dorsal surface of the
swim bladder and peritoneum extending to the
gut near the vent. Transitional larvae intensify
the two stripes and the mid lateral stripe
extends forward to the tip of the upper jaw
and the upper stripe continues irregularly
backwards to the dorsal caudal peduncle. A
third short stripe develops on top of the
head from the braincase towards the origin
of the dorsal fin. Smaller melanophores develop
between the anterior portions of the main
two stripes and just above the anal fin, as
well as in a series of fine patches just below
the mid-lateral stripe. The larval melanophores
on the pectoral and pelvic fin ray membranes
disappear rapidly, those between the dorsal-fin
spines disappear last. Melanophores appear
on the iris at about 3, 7, 9, and 10 o'clock. |
|
|
|
| Diplectrum
bivittatum larva |
| 12.7 mm SL |
| San Blas, Panama,
SB84-627 |
|
 |
| |
 |
| Diplectrum
bivittatum transitional larva |
| 12.8 mm SL |
| San Blas, Panama,
SB86-1001 |
|
 |
| |
 |
| |
 |
| |
 |
| Diplectrum
bivittatum late transitional
larva |
| 12.3 mm SL |
| San Blas, Panama,
SB86-1002 |
|
 |
| |
 |
| Diplectrum
bivittatum late transitional
larva |
| 12.9 mm SL |
| San Blas, Panama,
SB86-1008 |
|
 |
|
|
|
|
| |
|
|
|
|
| |
| Subfamily
Grammistinae |
| Rypticus |
|
|
| |
|
|
| Diagnosis:
Reduced spinous dorsal fin with only 2-4 dorsal-fin
spines and no obvious anal-fin spines indicates
the soapfishes of Rypticus.
There are six regional species with broadly
overlapping fin-ray counts. They can be separated
somewhat by the number of dorsal-fin spines:
R. saponaceus
and R. randalli
have 3 dorsal-fin spines, R.
subbifrenatus has 3 or 4 dorsal-fin
spines, and the remaining species have only
two dorsal-fin spines: the widespread
R. bistrispinus and R.
bornoi (= R.
macrostigmus) and R.
maculatus from US waters and the GOM
only. |
|
| Description:
Body thick and moderately wide with a medium
round eye and large terminal mouth. Pectoral-fin
rays very long, reaching well past the vent,
pelvic fins short, dorsal-fin base long and
anal-fin base medium length, caudal peduncle
relatively narrow and short. First dorsal-fin
spine prominent and covered in soft tissue,
subsequent spines very short. Fins generally
covered in soft tissue membranes. Very few
melanophores, typically only along the pectoral-fin
rays. |
|
|
|
| Rypticus
larva |
| 9.6 mm SL |
| San Blas, Panama,
SB83-168 |
|
 |
| Rypticus
larva |
| 10.5 mm SL |
| San Blas, Panama,
SB83-169 |
|
 |
|
|
|
|
|
|
|
|
| Diagnosis:
|
|
| Description:
Body relatively thick, wide, and short
with a large eye and large terminal mouth.
pectoral-fin rays very long, pelvic fins short,
dorsal-fin base relatively long and anal-fin
base medium, caudal peduncle wide and short.
Fins generally covered in soft tissue membranes.
Very few melanophores, typically only along
the pectoral-fin rays. |
|
|
| |
|
|
|
|
|
|
| |
| Subfamily
Pseudogrammatinae |
| Pseudogramma gregoryi |
|
|
|
|
|
|
|
|
| Diagnosis:
Modal fin-ray counts of D-VII,18-19 A-III,15-16
indicate Pseudogramma
gregoryi. (U) |
|
| Description:
Body relatively thin, somewhat long and narrow
with a large eye and large terminal mouth.
pectoral-fin rays very long, pelvic fins short,
dorsal and anal-fin bases relatively long,
caudal peduncle wide and short. |
|
| FAMILY
GRAMMATIDAE |
| Gramma loreto |
|
|
|
|
|
|
|
|
|
|
| Gramma
loreto larva |
| 10.1 mm SL |
| x |
|
 |
| Gramma
loreto larva |
| 10.1 mm SL |
| x |
|
 |
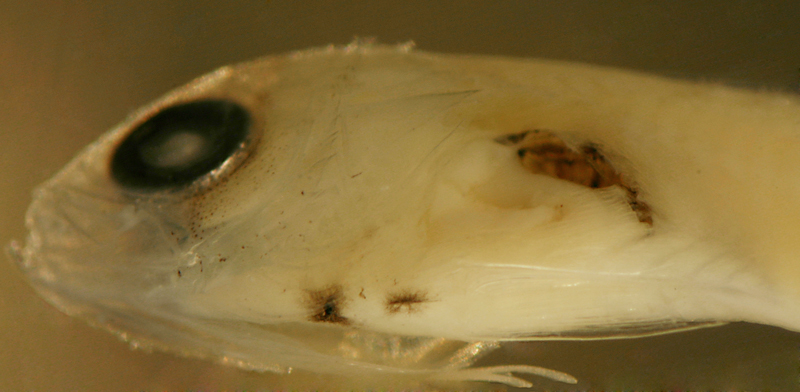
| Gramma
loreto larva |
| 10.1 mm SL |
| x |
|
 |
| Gramma
loreto larva |
| 10.2 mm SL |
| x |
|
 |
| Gramma
loreto larva |
| 10.8 mm SL |
| x |
|
 |
|
|
|
|
|
|
|
| |
|
| |
 |
|
All contents © copyright 2006-2014
All rights reserved
www.coralreeffish.com by
Benjamin Victor
|
|
|
|
| |
|
| |