|
|
|
| The
parrotfishes are abundant around Caribbean
coral reefs, especially in beds of seagrass
or macroalgae. They are typically the
predominant vertebrate herbivores on
and off of the reef. The taxonomy of
scarids in the region is relatively
simple: there are four genera, but virtually
all of the species belong to two large
genera Scarus
and Sparisoma.
The two remaining species comprise the
monotypic Cryptotomus
roseus and Nicholsina
usta, the latter with a sibling
species in the eastern Pacific. |
| |
| Larval
scarids share most of their basic features
with their labrid
relatives, such as long and continuous
dorsal and anal fins with slender spines,
a relatively wide caudal peduncle, stub-like
pelvic fins, a pointed snout and small
terminal mouth, typically light markings
and no spines on the head. They can
be separated from larval labrids by
having a row of melanophores along or
beneath the base of the anal fin, typically
extending into the caudal peduncle.
A number of similar-appearing families
share the anal-fin row of melanophores,
but have many more dorsal and anal-fin
elements, usually twice as many in larval
labrisomids,
chaenopsids, tripterygiids,
and dactyloscopids.
The latter group of larvae also have
narrower caudal peduncles, larger mouths,
long pelvic fins, and the anal-fin row
of melanophores is right at the base
of the fin rays and not deep as in the
parrotfishes. |
| |
| The
parrotfish family is remarkably uniform
in many aspects and all species share
the invariant fin-ray count of D-IX,10
A-III,9. Given the morphological and
meristic consistency of the family,
especially within the two large genera,
DNA-sequence analyses are required for
identifications to the species level. |
| |
| Pre-transitional
scarid larvae can have eyes that are
a narrowed vertical oval, often markedly
so. This character is shared by larval
razorfishes of Xyrichtys
and some larval gobies.
The eye becomes fully round in larval
scarids just before the onset of transitional
markings. |
|
|
|
|
|
|
|
|
|
| Diagnosis:
Fin-ray counts of D-IX,10 A-III,9 are
shared by all Caribbean parrotfishes, however
a mode of 14-16 pectoral-fin rays indicates
Scarus. The remaining parrotfishes
Sparisoma,
Cryptotomus
roseus, and Nicholsina
usta all have 13 pectoral-fin rays,
while the similar-appearing wrasse Doratonotus
megalepis shares the median-fin-ray
count but has only 11-12 pectoral-fin rays.
There are six Caribbean Scarus species,
with some slight separation by pectoral-fin-ray
count: Scarus
iseri and S.
taeniopterus have 13-14 pectoral-fin
rays (modal 14), S.
vetula has 14, rarely 15, S.
coeruleus usually has 15, and S.
guacamaia and S. coelestinus
have 16 pectoral-fin rays. Separating the
larvae of those species with overlapping pectoral-fin
ray counts requires DNA sequencing.
Scarus iseri (often mistakenly cited
as Scarus iserti)
vastly outnumbers the other species at most
Caribbean locations. |
|
| Analogues:
Wider-bodied Scarus larvae can resemble
larval Doratonotus
megalepis, but the latter do not have
the row of melanophores along the base of
the anal fin. Transitional Scarus
larvae lose their anal-fin base melanophores,
and then the two taxa can be separated by
the pattern of transitional melanophores on
the head. |
|
| Description:
Body relatively thin, typically long and narrow
with a large eye and a terminal small mouth
(some individuals are more wide-bodied and
leaf-shaped and are presumably approaching
transition). Pectoral fins short and pelvic
fins stubs in pre-transitional larvae. Dorsal
and anal-fin bases relatively long, caudal
peduncle short and relatively wide. Lightly
marked; an irregular row of up to 12 melanophores
along or beneath the base of the anal fin
extending into the caudal peduncle. There
is marked variability in the line-up of this
row of melanophores. The typical pattern for
the first seven melanophores is the first
three after the vent are deep in the body
and not along the base of the anal-fin rays
and the next four are located at the base
of the fin rays and can be expanded and appear
larger than the rest (i.e. 3+4, sometimes
4+3). The next in the row is usually well
above the fin base and the then last four
are in a row starting near the base of the
last anal-fin ray slanting up into the caudal
peduncle musculature. Many individuals are
missing some of the row of melanophores, some
show as few as five. There is a variable row
(from none to 10, occasionally 20 or more)
of tiny melanophores along the dorsal midline
of the caudal peduncle (often can be slightly
offset and variably paired), starting just
behind the base of the last dorsal-fin ray.
Melanophores occur internally around the gut
near the vent and often there is an additional
melanophore around the gut well above the
vent along the posterior peritoneum. Series
of transitional larvae show development of
the eye from a narrowed vertical oval, usually
tilted forward with a sometimes marked posterior-inferior
extension of the iris, to large and round
with a relatively small pupil at transition.
Many pre-transitional larvae have a ventral
indentation in the iris, sometimes with a
dorsal indentation as well, and rare individuals
have the narrowed eyes clearly tilted backward.
Some transitional individuals develop a particularly
bulbous eyeball with a tiny pupil. Transitional
larvae develop a scattering of tiny melanophores
on the top of the head along with a bar of
iridophores slanting upward from the back
of the eye and in a stripe from the eye to
the pectoral fin base. On the body, large
leukophores develop along the base of the
dorsal and anal fins and three leukophore
patches appear at the base of the upper, mid,
and lower segmented caudal fin rays. The larval
row of melanophores along the anal-finbase
disappears. Transitional recruits develop
additional melanophores densely covering the
top of the braincase and a scattering on the
snout and along the upper jaw and a stripe
angling upward from the rear of the eye. Additional
melanophores develop in two dense stripes
along the body, wider below the lateral midline
than above. Stripes develop later along the
base of the dorsal and anal fins. |
|
|
|
| Scarus
iseri larva |
| 6.7 mm SL |
| note narrowed
eye and wide body |
| San Blas, Panama,
SB86-506 |
|
 |
| |
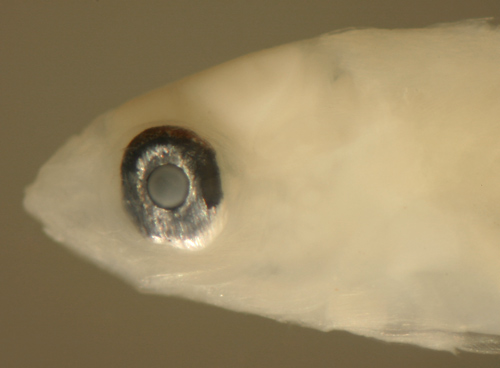 |
| Scarus
iseri larva |
| 6.3 mm SL |
| San Blas, Panama,
SB86-1103 |
|
 |
| Scarus
iseri larva |
6.2 mm SL |
| note iris indentation
and wide body |
| San Blas, Panama,
SB87-201 |
|
 |
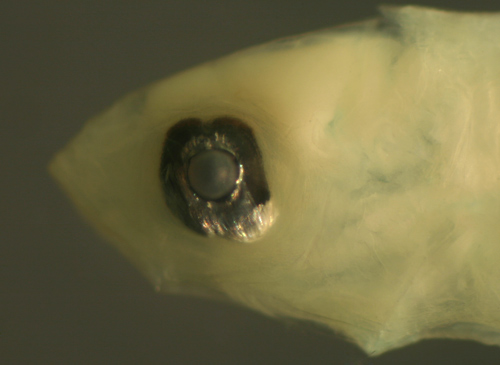
| Scarus
iseri larvae |
| 6.5 and 6.3 mm
SL |
note variant
above with narrow eye
tilted backwards, variation in anal
fin
base melanophores |
| San Blas, Panama,
SB87-201 |
|
 |
| |
 |
| |
 |
| Scarus
iseri larva |
| 6.7 mm SL |
| missing many
melanophores in anal-finrow |
| San Blas, Panama,
SB81-002 |
|
 |
| Scarus
iseri early transitional larva |
| 6.8 mm SL |
| fully-round eye
before transitional markings |
| San Blas, Panama,
SB86-516 |
|
 |
| Scarus
iseri larva |
| 6.9 mm SL |
| San Blas, Panama,
SB86-516 |
|
 |
| Scarus
iseri larva |
| 7.0 mm SL |
variant with
numerous paired
melanophores on caudal peduncle |
| San Blas, Panama,
SB86-808 |
|
 |
| Scarus
iseri transitional larva |
| 6.9 mm SL |
| San Blas, Panama,
SB81-001 |
|
 |
| |
 |
| Scarus
sp. transitional recruit |
| 6.9 mm SL |
| anterior body
melanophores contracted |
| Barbados 81104,
Henri Valles |
|
 |
| |
 |
| Scarus
iseri transitional larva |
| 6.9 mm SL |
| San Blas, Panama,
SB81-001 |
|
 |
|
|
|
|
|
|
|
|
| Diagnosis:
Fin-ray counts of D-IX,10 A-III,9
are shared by all Caribbean parrotfishes,
however pectoral-fin ray counts divide parrotfishes
into two groups: Sparisoma,
Cryptotomus
roseus, and Nicholsina
usta all have 13 pectoral-fin rays,
while Scarus
have a mode of 14-16 pectoral-fin rays (the
wrasse Doratonotus
megalepis also shares the median-fin
ray count but has only 11-12 pectoral-fin
rays). The larvae of the seven Caribbean species
of the genus Sparisoma
(S.
atomarium, S.
aurofrenatum, S.
chrysopterum, S.
radians, S.
rubripinne, and S.
viride; with S.
griseorubra in Venezuela) are likely
indistinguishable from each other and separation
requires DNA sequencing. Cryptotomus
roseus can be excluded since its larvae
appear to be missing the characteristic lateral
melanophore on the body on each side just
above the pelvic-fin insertion. Species differences
that occur after transition are noted in the
individual species descriptions that follow.
Larval Nicholsina
usta cannot be excluded from the type
until those larvae are identified (adults
of the species are not found at the collection
site in Panama). (R) |
|
| Note:
the colors, patterns, and markings
of juvenile Sparisoma
are remarkably variable and changeable with
habitat and mood, indeed juveniles can change
from blotchy to striped to bars to uniformly
green as one observes them in the field. Background
color varies widely from reddish to salmon
to yellow to green. Overall, juvenile Sparisoma
show variations in degree of the same general
pattern of blotches and body stripes (which
often break up into spots) that are characteristic
of the genus. Nevertheless, there are some
diagnostic markings in small juveniles that
can help to separate the species. DNA sequencing
is underway at present to identify the species-specific
features of juvenile markings in this genus. |
|
| Analogues:
|
|
| Description:
Body relatively thin, long and narrow with
a large eye and a terminal small mouth. Pectoral
fins short and pelvic fins usually stubs.
Dorsal and anal-finbases relatively long,
caudal peduncle short and somewhat narrow.
Melanophores consist of one on the body on
each side just above the pelvic-fin insertion,
internally around the gut near the vent, and
in a row of 13 discrete round melanophores
along or often below the base of the anal-fin
and extending into the caudal peduncle (some
larvae have only 12, missing the first in
the series). The melanophores in the row after
the last anal-fin ray are not at the ventral
midline but well into the caudal peduncle
musculature. Series of transitional larvae
show development of the eye from a narrowed
vertical oval tilted forward (sometimes backwards
or no tilt) with a small posterior-inferior
extension of the iris to larger and round
with a smaller pupil at and after transition
(eye usually becomes fully round just before
transitional markings appear). Many pre-transitional
larvae have a marked ventral indentation in
the iris. A small fraction of larval collections
show individuals with head and eye abnormalities
including exophthalmos and a pronounced bulbous
head. It is unclear whether these are artifacts
of collection or true abnormalities. Some
transitional larvae first develop two prominent
leukophore patches above and below the midline
at the base of the segmented caudal fin rays
and then the anal-fin row of melanophores
start to disappear. Others acquire melanophores
first, typically around the eye and on the
first dorsal and anal-fin elements and the
pelvic fin. Early transitional larvae or recruits
develop tiny leukophores along the first dorsal
spines and then in patches spaced along the
base of some dorsal and anal-fin rays. A central
patch of leukophores starts to develop on
the base of the caudal fin rays and then variably
coalesces with the upper and lower patches
into a white bar. Surface melanophores appear
scattered over the top of the head and anterior
upper body and often in patches along the
base of the anal-fin rays (these patches of
tiny surface melanophores are distinct from
the large larval melanophores). Melanophores
also develop along the first dorsal spines
and the proximal pelvic-fin rays with leukophores
on the more distal portions of the spines
and rays. Mid-transitional larvae or recruits
continue to develop a bar of melanophores
below the front of the eyeball and a stripe
forward of the eye which branches down to
the middle of the lower jaw and up across
the mid-upper jaw to the tip of the lower
jaw. Melanophores develop in two upward-angled
stripes from the top and rear of the eyeball
and a downward-angled stripe develops rearward
from the eye across the cheek. A stripe of
iridophores develops slanting upward from
the back of the eye and in a stripe slanting
down across the cheek just above the melanophore
stripe. Melanophores continue to develop in
discrete patches along the base of the dorsal
fin and intensify along the base of the anal
fin. Markings on the body develop from anterior
to posterior, particularly along the lateral
midline. The characteristic larval melanophore
over the pelvic-fin insertion is lost. Late
transitional recruits show a variety of patterning
on the lateral body, mostly in irregular patches
and bars but with variants showing 1) additional
fine melanophores outlining myomeres, 2) a
uniform spotting of small melanophores (S.
viride only ?), or 3) an irregular
mid-lateral stripe. There appear to be few
consistent differences in this pattern among
species until the juvenile stage (about 12
to 14 mm SL) when some distinctions start
to develop. Sparisoma
recruits are notable for expanding
first in body depth and girth for the first
two weeks or so after settlement and then
beginning to increase in length. |
|
|
|
| Sparisoma
sp. larva |
| 9.3 mm SL |
| San Blas, Panama,
SB84-522 |
|
 |
| |
 |
| Sparisoma
sp. larva |
| 9.2 mm SL |
| San Blas, Panama,
SB86-825 |
|
 |
| Sparisoma
sp. larva |
| 9.9 mm SL |
| characteristic
rear melanophore pattern |
| San Blas, Panama,
SB86-413 |
|
 |
| Sparisoma
sp. larva |
| 9.1 mm SL |
| narrowed eye,
DNA ID pending |
| San Blas, Panama,
SB86-825 |
|
 |
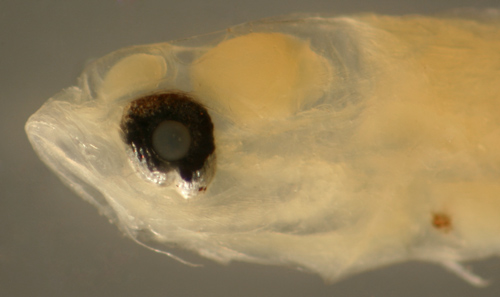
| Sparisoma
sp. larva |
| 8.7 mm SL |
| iris extension,
DNA ID pending |
| San Blas, Panama,
SB82-020 |
|
 |
| Sparisoma
sp. larva |
| 9.1 mm SL |
| round eye before
any transitional markings |
| San Blas, Panama,
SB87-117 |
|
 |
| Sparisoma
sp. transitional larva |
| 9.3 mm SL |
loss of some
anal row melanophores
DNA ID pending |
| San Blas, Panama,
SB86-422 |
|
 |
| |
 |
| Sparisoma
sp. early transitional larva |
| 9.9 mm SL |
eye already round
with a small pupil
DNA ID pending |
| Barbados 100802,
Henri Valles |
|
 |
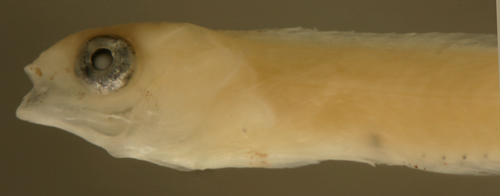
| Sparisoma
sp. transitional larva |
| 9.8 mm SL |
head and fin
melanophores developing
DNA ID pending |
| San Blas, Panama,
SB81-037 |
|
 |
| Sparisoma
sp. transitional larva |
| 9.9 mm SL |
head and fin
melanophores developing
DNA ID pending |
| San Blas, Panama,
SB86-506 |
|
 |
| Sparisoma
sp. late transitional larva |
| 10.0 mm SL |
| San Blas, Panama,
SB81-002 |
|
 |
| Sparisoma
sp. early transitional recruit |
| 9.2 mm SL |
mostly leukophores,
a fine scattering of
anterior and head melanophores
DNA ID pending |
| Barbados 81104,
Henri Valles |
|
 |
| Sparisoma
viride early transitional recruit |
| 9.9 mm SL |
lightly marked
anteriorly, captured with
S. viride series, DNA ID pending |
| SB81-062 |
|
 |
| Sparisoma
sp. early transitional recruit |
| 9.1 mm SL |
variant with
irregular lateral stripe and
abdominal midline melanophore patch
DNA ID pending |
| Barbados 81104,
Henri Valles |
|
 |
| Sparisoma
sp. mid transitional recruit |
| 9.9 mm SL |
variant with
anterior markings and anal fin
base melanophore patches
DNA ID pending |
| Barbados 62903,
Henri Valles |
|
 |
| |
 |
| Sparisoma
sp. mid transitional recruit |
| 9.0 mm SL |
| Barbados 81104,
Henri Valles |
|
 |
| Sparisoma
sp. late transitional recruit |
| 9.8 mm SL |
variant with
prominent myomere outlining
DNA ID pending |
| Barbados 62903,
Henri Valles |
|
 |
| |
 |
| Sparisoma
spp. early transitional recruits |
with head and
eye abnormalities
including exophthalmos and bulbous head
DNA ID pending |
| Barbados V0553,
Henri Valles |
|
 |
|
|
|
|
| |
|
|
|
|
Diagnosis:
The larvae of all Sparisoma
may well be identical, and DNA sequencing
is required to identify species. Transitional
recruits develop the basic markings probably
shared by all members of the genus, but
small juveniles of Sparisoma
acquire distinct patterns that separate
most, if not all, regional species. S.
viride diverges from the remainder
of the genus the earliest, with some individuals
smaller than 10 mm SL showing a distinct
pattern of markings, in particular an undivided
prominent white bar on the caudal fin base.
|
|
| Description:
This type shares the characteristic
markings of larval and transitional Sparisoma.
Recruits become distinct early on when the
leukophores on the base of their caudal fin
coalesce into a distinct white bar and they
develop rows of round white spots, with the
two above the pectoral fin most visible. Characteristically,
there are no melanophores extending into the
white bar (at least until about 15 mm SL,
but by then the white bar and rows of white
spots are clearly prominent). |
|
|
|
| Sparisoma
viride recruit |
| 9.6 mm SL |
| variant with
early spot/bar pattern |
| Barbados 62903,
Henri Valles |
|
 |
| Sparisoma
viride recruit |
| 9.7 mm SL |
| light markings |
| San Blas, Panama,
SB81-077 |
|
 |
| |
 |
| Sparisoma
viride recruit |
| 11.0 mm SL |
| San Blas, Panama,
SB81-077 |
|
 |
| |
 |
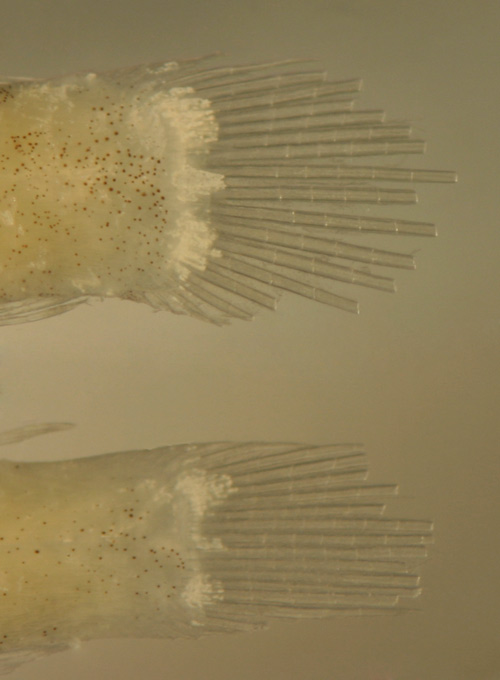
| Sparisoma
viride recruits |
| 11.0 mm and 9.7
mm SL |
| note no melanophores
into caudal bar |
| DNA ID pending |
| San Blas, Panama,
SB81-077 |
|
 |
| Sparisoma
viride juvenile |
| 12.3 mm SL |
| San Blas, Panama,
SB81-062 |
|
 |
| Sparisoma
viride juveniles |
| 14.9 and 13.0
mm SL |
| San Blas, Panama,
SB81-060 |
|
 |
| Sparisoma
viride juvenile |
| 15.6 mm SL |
after 15 mm SL
there is some extension
of melanophores into caudal bar |
| San Blas, Panama,
SB80-091 |
|
 |
|
|
|
|
|
|
|
|
|
Diagnosis:
The larvae of all Sparisoma
may well be identical, and DNA sequencing
is required to identify species. Transitional
recruits develop the basic markings probably
shared by all members of the genus, but
small juveniles of Sparisoma
acquire distinct patterns that separate
most, if not all, regional species.
The DNA sequence of the juvenile specimen
from Noronha in Brazil confirms that it
is S. radians
(Bernardi et al 2005), even though it displays
a peculiar pattern of markings. S.
atomarium may be indistinguishable
from S. radians
when juvenile specimens are found in the
same habitat.
|
|
| Description:
This type shares the characteristic markings
of larval and transitional Sparisoma.
Recruits become distinct from S.
viride early as melanophores extend
onto the base of the central caudal fin rays
and divide the light bar on the tail. The
melanophores extending into the caudal bar
extend further below the midline than above
(vs. equal above and below in S.
chrysopterum/rubripinne). Small juveniles
tend to have dark patches along the lateral
midline mostly below the level of the lateral
line and do not develop an obvious white tail
bar. Some individuals develop a marked bicolor
pattern of light above the lateral line and
dark below. Later juveniles are variably mottled
with some light striping and spotting and
are only identified by process of exclusion
(or DNA sequence analysis). Individuals from
Noronha in Brazil show a pattern of reduced
body markings and intensified black markings
on the fins. |
|
|
|
| Sparisoma
radians juvenile |
| 13.3 mm SL |
| San Blas, Panama,
SB80-101 |
|
 |
| |
 |
| Sparisoma
radians juvenile |
| 16.5 mm SL |
| San Blas, Panama,
SB81-027 |
|
 |
| Sparisoma
radians juvenile |
| 17.8 mm SL |
| San Blas, Panama,
SB80-105 |
|
 |
| |
 |
| Sparisoma
radians juvenile |
| 18.0 mm SL |
| DNA ID confirmed |
| Noronha, Brazil
FN01 |
|
 |
| Sparisoma
sp. juvenile |
| 13.9 mm SL |
| San Blas, Panama,
SB80-102 |
|
 |
|
|
|
|
| |
|
|
|
|
Diagnosis:
The larvae of all Sparisoma
may well be identical, and DNA sequencing
is required to identify species. Transitional
recruits develop the basic markings probably
shared by all members of the genus, but
small juveniles of Sparisoma
acquire distinct patterns that separate
most, if not all, regional species.
S. chrysopterum
and S. rubripinne
may have a similar appearance as juveniles.
|
|
|
Description:
This type shares the characteristic markings
of larval and transitional Sparisoma.
Recruits become distinct from S.
radians and S.
viride as melanophores extend onto
the base of the caudal fin and divide the
light bar on the tail. The tail melanophores
extending into the caudal bar are roughly
equal both above and below the midline and
the bar is still clearly white. Juveniles
are variably marked, but typically develop
an alternating pattern of white and dark
bars.
|
|
|
|
| Sparisoma
chrysopterum + juvenile |
| 14.0 mm SL |
| DNA
ID pending |
| San Blas, Panama,
SB81-062 |
|
 |
| |
 |
| Sparisoma
chrysopterum + juvenile |
| 13.3 mm SL |
| San Blas, Panama,
SB80-103 |
|
 |
| |
 |
|
|
|
|
|
|
|
|
|
|
Diagnosis:
Fin-ray counts of D-IX,10 A-III,9
are shared by all Caribbean parrotfishes,
however pectoral-fin ray counts divide parrotfishes
into two groups: Sparisoma,
Cryptotomus roseus, and Nicholsina
usta all have 13 pectoral-fin rays,
while Scarus
have a mode of 14-16 pectoral-fin rays (the
wrasse Doratonotus
megalepis also shares the median
fin-ray count but has 11-12 pectoral-fin
rays). This larval type develops into Cryptotomus
roseus when raised in captivity,
but the demarkation between C.
roseus and Sparisoma
is unclear. Larval Nicholsina
usta cannot be excluded from the
type until those larvae are identified (adults
of the species are not found at the collection
site in Panama). (R)
|
|
| Analogues:
C. roseus
is primarily identified by the absence of
the characteristic lateral melanophore of
Sparisoma
in a pre-transitional larva (does not apply
to transitional larvae). Additional characters
that may assist are the loss (or fading out)
of one or more of the last few anal row melanophores,
which correlates well with no lateral melanophore
(also only applicable to pre-transitional
larvae). Most C.
roseus larvae do have fewer than 13
melanophores in the anal-fin row. Lastly,
the snout is usually sharply-pointed in this
larval type. Unfortunately, transitional Sparisoma
larvae can lose their lateral melanophore
and show a reduced complement of anal row
melanophores: thus the distinction becomes
difficult at early transition before the metamorphic
melanophore pattern starts. Furthermore, there
is the possibility that some rare pre-transition
Sparisoma
do lack the lateral melanophore and/or the
full 13 anal row melanophores (some larvae
have 12 in the row, but are missing the first
and not the last). DNA sequence analyses underway
at present should resolve this potential overlap.
|
|
|
Description:
Body relatively thin, long and narrow with
a large eye and a pointed snout and a terminal
small mouth. Pectoral fins medium. Pelvic
fins very short. Dorsal and anal-fin bases
relatively long, caudal peduncle short and
somewhat narrow. Melanophores occur internally
around the gut near the vent, and in a row
of 11, 12, or occasionally 13 (but rule
out Sparisoma
when 13) discrete round melanophores along
the base of the anal fin and extending into
the caudal peduncle (often missing the last
in the series). The melanophores in the
row after the last fin ray are not at the
ventral midline but can be well into the
caudal peduncle musculature. Series of transitional
larvae show development of the eye from
a narrowed vertical oval tilted forward
with a small posterior-inferior extension
of the iris to much larger and round at
and after transition. Many pre-transitional
larvae have a marked ventral indentation
in the iris. Transitional larvae develop
a few scattered melanophores on the top
of the head and two arcs from the mid and
upper eye across the top of the head (transitional
Sparisoma
have a similar upper arc but do not have
the arc starting at the mid-eye).
|
|
|
|
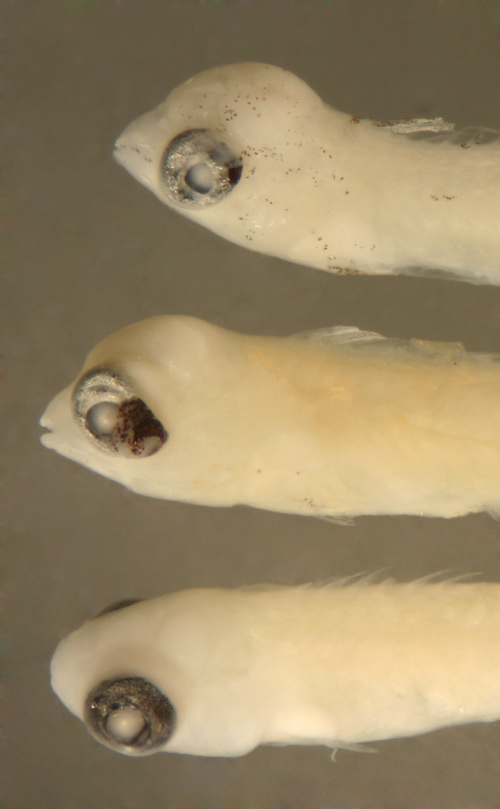
| Cryptotomus
roseus larva |
| 8.8 mm SL |
| note 13 anal-fin
row melanophores |
| San Blas, Panama,
SB86-604 |
|
 |
| Cryptotomus
roseus larva |
| 8.3 mm SL |
| note 12 anal-fin
row melanophores |
| San Blas, Panama,
SB86-422 |
|
 |
| |
 |
| Cryptotomus
roseus larva |
| 9.5 mm SL |
note reduced
row of caudal
peduncle melanophores |
| San Blas, Panama,
SB86-1028 |
|
 |
Sparisoma
larva, at top
vs. Cryptotomus
roseus larvae below |
| 9.2, 9.2, and
9.3 mm SL |
note reduced
rows of caudal
peduncle melanophores |
| San Blas, Panama,
SB82-020 |
|
 |
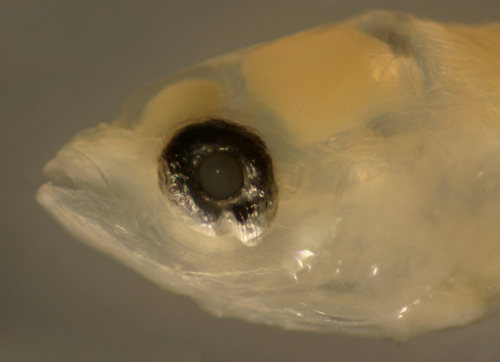
| Cryptotomus
roseus larva |
| 9.2 mm SL |
| San Blas, Panama,
SB82-020 |
|
 |
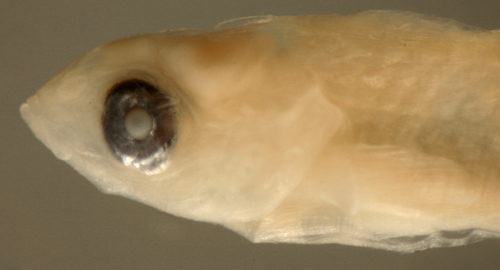
| Cryptotomus
roseus larva |
| 7.8 mm SL |
| San Blas, Panama,
SB81-047 |
|
 |
| Cryptotomus
roseus transitional larva |
| 8.4 mm SL |
| note 13 anal-fin
row melanophores |
| San Blas, Panama,
SB86-608 |
|
 |
| |
 |
| Cryptotomus
roseus recruit |
| 12.4 mm SL |
| large round eye |
| San Blas, Panama,
SB80-093 |
|
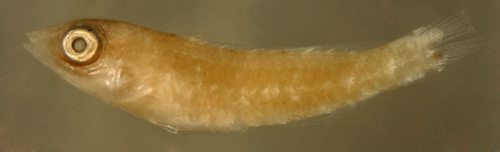 |
| Sparisoma
vs. Cryptotomus
roseus |
| 9.8 mm SL |
the lateral melanophore
is absent,
but all melanophores are very faint |
| San Blas, Panama,
SB86-422 |
|
 |
| |
 |
|
|
|
|
| |
|
|
|
|
Diagnosis:
Fin-ray counts of D-IX,10 A-III,9
are shared by all Caribbean parrotfishes,
however pectoral-fin ray counts divide parrotfishes
into two groups: Sparisoma,
Cryptotomus
roseus, and Nicholsina usta
all have 13 pectoral-fin rays, while Scarus
have a mode of 14-16 pectoral-fin rays (the
wrasse Doratonotus
megalepis also shares the median
fin-ray count but has 11-12 pectoral-fin
rays). Larval and transitional markings
are unknown, but based on the appearance
of the eastern Pacific sibling species,
Nicholsina denticulata, recruits
have a distinct uniformly dark pattern distinct
from C.
roseus and Sparisoma
|
|
|
Description:
|
|
|
|
| Nicholsina
denticulata recruit |
| 11.9 mm SL |
| Baja California,
Mexico B01-628ss |
|
 |
| |
 |
|
|
| |
|
 |
|
All contents © copyright
2006-2013
All rights reserved
www.coralreeffish.com
by Benjamin Victor
|
|
|
|
|
|
|
|